Abstract
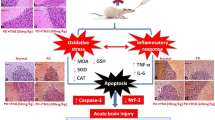
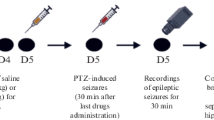
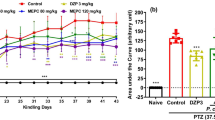
Methylene blue (MethyB) was shown to protect against different brain pathologies. In this study, the effect of acute administration of MethyB on epileptic seizures, brain oxidative stress, and neuronal injury induced in rats by pentylenetetrazole (PTZ) was studied. Rats received repeated intraperitoneal (i.p.) injections of PTZ until the development of status epilepticus and were i.p. treated once with MethyB at doses of 50 or 100 mg/kg, 30 min prior to the first PTZ injection. Results indicated that compared with the PTZ control group, the administration of MethyB at 50 and 100 mg/kg decreased the mean seizure score over the study period by 48.8% and 55.1%, respectively. The development of myoclonic jerks and generalized tonic-clonic seizures was inhibited by 90.0–85.0% and 45.4–56.3%, respectively, compared with the PTZ control group. The mean latency to develop status epilepticus increased by 43.1% after treatment with MethyB at 100 mg/kg. MethyB had no significant effect on the mean threshold dose of PTZ required to reach status epilepticus. MethyB showed inhibitory effects on the increase in brain lipid peroxidation (malondialdehyde), and nitric oxide induced PTZ by 16.7–24.0% and 25.2–32.6%, respectively. Meanwhile, the decrease in reduced glutathione and paraoxonase-1 (PON-1) activity and the increase in nuclear factor-kappa B (NF-κB) in the brain of PTZ-treated rats were attenuated by MethyB. Brain acetylcholinesterase (AChE) concentration decreased by PTZ and showed further decrements after treatment with either dose of MethyB. PTZ caused neuronal atrophy in the hippocampus and cerebral cortex and decreased neuronal size and number in the substantia nigra. These pathological changes were prevented by MethyB given at 100 mg/kg. These data indicate that treatment with MethyB inhibits the PTZ-induced seizures in rats and increases the latency to develop status epilepticus. In addition, MethyB decreases brain oxidative stress and protects against neuronal atrophy in this experimental model of generalized epilepsy.







Similar content being viewed by others
References
Abdel-Salam OME, Omara EA, Youness ER, Khadrawy YA, Mohammed NA, Sleem AA (2014) Rotenone-induced nigrostriatal toxicity is reduced by methylene blue. J Neuro-Oncol 2:65–80
Abdel-Salam OME, Sleem AA, Sayed MAEM, Khadrawy YA, Morsy FA 2018a. Cannabis sativa increases seizure severity and brain lipid peroxidation in pentylenetetrazole-induced kindling in rats. Biomed Pharmacol J;11(3)
Abdel-Salam OME, Sleem AA, Sayed MAEM, Youness ER, Shaffie N 2019 Neuroprotective effects of low dose anandamide in pentylenetetrazole-induced kindling in rats. Biomed Pharmacol J;12(1)
Abdel-Salam OME, Sleem AA, Youness ER, Mohammed NA, Shaffie N, Yassen NN (2018b) Neuro- and hepatoprotective effects of methylene blue in rats treated with lipopolysaccharide endotoxin. React Oxygen Species 6(17):325–337
Abdel-Salam OME, Youness ER, Esmail RSE, Mohammed NA, Khadrawy YA, Sleem AA (2016a) Methylene blue as a novel neuroprotectant in acute malathion intoxication. React Oxygen Species 1(2):165–177. https://doi.org/10.20455/ros.2016.821
Abdel-Salam OME, Youness ER, Morsy FA, Yassen NN, Mohammed NA, Sleem AA (2016b) Methylene blue protects against toluene-induced brain damage: involvement of nitric oxide, NF-κB, and caspase-3. React Oxygen Species 2(5):371–387
Acon-Chen C, Koenig JA, Smith GR, Truitt AR, Thomas TP, Shih TM (2016) Evaluation of acetylcholine, seizure activity and neuropathology following high-dose nerve agent exposure and delayed neuroprotective treatment drugs in freely moving rats. Toxicol Mech Methods 26(5):378–388. https://doi.org/10.1080/15376516.2016.1197992
Aharoni S, Aviram M, Fuhrman B (2013) Paraoxonase 1 (PON1) reduces macrophage inflammatory responses. Atherosclerosis 228(2):353–361. https://doi.org/10.1016/j.atherosclerosis.2013.03.005
Akbas SH, Yegin A, Ozben T (2005) Effect of pentylenetetrazol-induced epileptic seizure on the antioxidant enzyme activities, glutathione and lipid peroxidation levels in rat erythrocytes and liver tissues. Clin Biochem 38(11):1009–1014
Alda M, McKinnon M, Blagdon R, Garnham J, MacLellan S, O’Donovan C et al (2017) Methylene blue treatment for residual symptoms of bipolar disorder: randomised crossover study. Br J Psychiatry 210(1):54–60. https://doi.org/10.1192/bjp.bp.115.173930
Alyu F, Dikmen M (2017) Inflammatory aspects of epileptogenesis: contribution of molecular inflammatory mechanisms. Acta Neuropsychiatr 29(1):1–16. https://doi.org/10.1017/neu.2016.47
Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H et al (2008) Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J 22(3):703–712. https://doi.org/10.1096/fj.07-9610com
Bal-Price A, Brown GC (2001) Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci 21(17):6480–6491
Banach M, Piskorska B, Czuczwar SJ, Borowicz KK (2011) Nitric oxide, epileptic seizures, and action of antiepileptic drugs. CNS Neurol Disord Drug Targets 10(7):808–819
Bradberry SM (2003) Occupational methaemoglobinaemia: mechanisms of production, features, diagnosis and management including the use of methylene blue. Toxicol Rev 22(1):13–27
Bruchey AK, Gonzalez-Lima F (2008) Behavioral, physiological and biochemical hormetic responses to the autoxidizable dye methylene blue. Am J Pharmacol Toxicol 3(1):72–79
Castellazzi M, Trentini A, Romani A, Valacchi G, Bellini T, Bonaccorsi G, et al. Decreased arylesterase activity of paraoxonase-1 (PON-1) might be a common denominator of neuroinflammatory and neurodegenerative diseases. Int J Biochem Cell Biol 2016; 81(Pt B):356–363. doi: https://doi.org/10.1016/j.biocel.2016.06.008
Chang SJ, Yu BC (2010) Mitochondrial matters of the brain: mitochondrial dysfunction and oxidative status in epilepsy. J Bioenerg Biomembr 42(6):457–459. https://doi.org/10.1007/s10863-010-9317-4
Clifton J,2nd Leikin JB. Methylene blue. Am J Ther 2003; 10(4):289–291
Corda MG1, Orlandi M, Giorgi O. Decrease in GABAA receptor function induced by pentylenetetrazol kindling in the rat: role of N-methyl-D-aspartate (NMDA) receptors. Adv Biochem Psychopharmacol. 1992;47:235–247
Cui ZQ, Li WL, Luo Y, Yang JP, Qu ZZ, Zhao WQ (2018) Methylene blue exerts anticonvulsant and neuroprotective effects on self-sustaining status epilepticus (SSSE) induced by prolonged basolateral amygdala stimulation in Wistar rats. Med Sci Monit 24:161–169
Dailey JW, Yan QS, Mishra PK, Burger RL, Jobe PC (1992) Effects of fluoxetine on convulsions and on brain serotonin as detected by microdialysis in genetically epilepsy-prone rats. J Pharmacol Exp Ther 260(2):533–540
Dhir A 2012. Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr Protoc Neurosci;Chapter 9:Unit9.37. doi: https://doi.org/10.1002/0471142301.ns0937s58
Dibaj P, Zschüntzsch J, Steffens H, Scheffel J, Göricke B, Weishaupt JH et al. (2010) Influence of methylene blue on microglia-induced inflammation and motor neuron degeneration in the SOD1G93A model for ALS. PLoS One 7(8):e43963. https://doi.org/10.1371/journal.pone.0043963
Drury RVA, Walligton EA (1980) Carltons histological techniques, 5th edn. Oxford University Press, New York, Pronto
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Erdoğan F, Gölgeli A, Arman F, Ersoy AO (2004) The effects of pentylenetetrazole-induced status epilepticus on behavior, emotional memory, and learning in rats. Epilepsy Behav 5(3):388–393
Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(7):829–37, 37a–37d. https://doi.org/10.1093/eurheartj/ehr304
Grubb KJ, Kennedy JL, Bergin JD, Groves DS, Kern JA (2012) The role of methylene blue in serotonin syndrome following cardiac transplantation: a case report and review of the literature. J Thorac Cardiovasc Surg 144(5):e113–e116. https://doi.org/10.1016/j.jtcvs.2012.07.030
Haagen L, Brock A (1992) A new automated method for phenotyping arylesterase (EC 3.1.1.2) based upon inhibition of enzymatic hydrolysis of 4-nitrophenyl acetate by phenyl acetate. Eur J Clin Chem Clin Biochem 30(7):391–395
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18:685–716
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd edn. Clarendon Press, Oxford
Han D, Yamada K, Senzaki K, Xiong H, Nawa H, Nabeshima T (2000) Involvement of nitric oxide in pentylenetetrazole-induced kindling in rats. J Neurochem 74(2):792–798
Jelenković A, Jovanović MD, Bokonjić D, Maksimović M, Bosković B (2012) Influence of NG-nitro-L-arginine methyl ester on clinical and biochemical effects of methylene blue in pentylenetetrazole-evoked convulsions. Vojnosanit Pregl 69(6):481–487
Klamer D, Engel JA, Svensson L (2004) Phencyclidine-induced behaviour in mice prevented by methylene blue. Basic Clin Pharmacol Toxicol 94(2):65–72
La Du BN (1992) Human serum paraoxonase: arylesterase. Pergamon Press, New York, NY
Liu LM, Wang N, Lu Y, Wang WP (2019) Edaravone acts as a potential therapeutic drug against pentylenetetrazole-induced epilepsy in male albino rats by downregulating cyclooxygenase-II. Brain Behav 9(1):e01156. https://doi.org/10.1002/brb3.1156
Liu T, Zhang L, Joo D, Sun SC (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2:17023. https://doi.org/10.1038/sigtrans.2017.23
Mackness M, Mackness B (2013) Targeting paraoxonase-1 in atherosclerosis. Expert Opin Ther Targets 17(7):829–837. https://doi.org/10.1517/14728222.2013.790367
Maia GH, Brazete CS, Soares JI, Luz LL, Lukoyanov NV (2017) Serotonin depletion increases seizure susceptibility and worsens neuropathological outcomes in kainate model of epilepsy. Brain Res Bull 134:109–120. https://doi.org/10.1016/j.brainresbull.2017.07.009
Manghelli J, Brown L, Tadros HB, Munfakh NA (2015) A reminder of methylene blue's effectiveness in treating vasoplegic syndrome after onpump cardiac surgery. Tex Heart Inst J 42(5):491-4. https://doi.org/10.14503/THIJ-14-4470
Martins-Pinge MC, Araújo GC, Lopes OU (1999) Nitric oxide-dependent guanylyl cyclase participates in the glutamatergic neurotransmission within the rostral ventrolateral medulla of awake rats. Hypertension 34(4 Pt 2):748–751
Mayer B, Brunner F, Schmidt K (1993) Inhibition of nitric oxide synthesis by methylene blue. Biochem Pharmacol 45(2):367–374
Meissner PE, Mandi G, Coulibaly B, Witte S, Tapsoba T, Mansmann U, Rengelshausen J, Schiek W, Jahn A, Walter-Sack I, Mikus G, Burhenne J, Riedel KD, Schirmer RH, Kouyaté B, Müller O (2006) Methylene blue for malaria in Africa: results from a dose-finding study in combination with chloroquine. Malar J 5:84
Menini T, Gugliucci A (2014) Paraoxonase 1 in neurological disorders. Redox Rep 19(2):49–58. https://doi.org/10.1179/1351000213Y.0000000071
Miclescu A, Sharma HS, Martijn C, Wiklund L (2010) Methylene blue protects the cortical blood-brain barrier against ischemia/reperfusion-induced disruptions. Crit Care Med 38(11):2199–2206. https://doi.org/10.1097/CCM.0b013e3181f26b0c
Moshage H, Kok B, Huizenga JR, Jansen PL (1995) Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem 41(6 Pt 1):892–896
Müller T (1998) Methylene blue supravital staining: an evaluation of its applicability to the mammalian brain and pineal gland. Histol Histopathol 13(4):1019–1026. https://doi.org/10.14670/HH-13.1019
Naylor GJ, Martin B, Hopwood SE, Watson Y (1986) A two-year double-blind crossover trial of the prophylactic effect of methylene blue in manic-depressive psychosis. Biol Psychiatry 21(10):915–920
Nguyen SD, Sok DE (2004) Preferential inhibition of paraoxonase activity of human paraoxonase 1 by negatively charged lipids. J Lipid Res 45(12):2211–2220. https://doi.org/10.1194/jlr.M400144-JLR200
O’Leary JL, Petty J, Harris AB, Inukai J (1968) Supravital staining of mammalian brain with intra-arterial methylene blue followed by pressurized oxygen. Stain Technol 43(4):197–201
Oeckinghaus A, Ghosh S (2009) The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 1(4):a000034. https://doi.org/10.1101/cshperspect.a000034
Patsoukis N, Zervoudakis G, Panagopoulos NT, Georgiou CD, Angelatou F, Matsokis NA (2004) Thiol redox state (TRS) and oxidative stress in the mouse hippocampus after pentylenetetrazol-induced epileptic seizure. Neurosci Lett 357(2):83–86
Peter C, Hongwan D, Küpfer A, Lauterburg BH (2000) Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur J Clin Pharmacol 56(3):247–250
Petzer A, Harvey BH, Petzer JP (2014) The interactions of azure B, a metabolite of methylene blue, with acetylcholinesterase and butyrylcholinesterase. Toxicol Appl Pharmacol 274(3):488–493. https://doi.org/10.1016/j.taap.2013.10.014
Petzer A, Harvey BH, Wegener G, Petzer JP (2012) Azure B, a metabolite of methylene blue, is a high-potency, reversible inhibitor of monoamine oxidase. Toxicol Appl Pharmacol 258(3):403–409. https://doi.org/10.1016/j.taap.2011.12.005
Pfaffendorf M, Bruning TA, Batnik HD, van Zwieten PA (1997) The interaction between methylene blue and the cholinergic system. Br J Pharmacol 122(1):95–98
Pohle W, Becker A, Grecksch G, Juhre A, Willenberg A (1997) Piracetam prevents pentylenetetrazol kindling-induced neuronal loss and learning deficits. Seizure 6(6):467–474
Ramsay RR, Dunford C, Gillman PK (2007) Methylene blue and serotonin toxicity: inhibition of monoamine oxidase A (MAO A) confirms a theoretical prediction. Br J Pharmacol 152(6):946–951
Reiter RJ (1995) Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB L 9:526–533
Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, de Groot H (1994) Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids 59(6):383–388
Sayre LM, Perry G, Smith MA (2008) Oxidative stress and neurotoxicity. Chem Res Toxicol 21(1):172–188
Scharfman HE (2007) The neurobiology of epilepsy. Curr Neurol Neurosci Rep 7(4):348–354
Schirmer RH, Coulibaly B, Stich A et al (2003) Methylene blue as an antimalarial agent. Redox Rep 8:272–275
Sefil F, Kahraman I, Dokuyucu R, Gokce H, Ozturk A, Tutuk O, Aydin M, Ozkan U, Pinar N (2014) Ameliorating effect of quercetin on acute pentylenetetrazole induced seizures in rats. Int J Clin Exp Med 7(9):2471–2477
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82(2):291–295
Sies H, Stahl W, Sundquist AR (1992) Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann N Y Acad Sci 669:7–20
Solmaz V, Aksoy D, Yurtogulları S, Semiz M, Aydemir E, Erbas O (2016) The effects of methylene blue and tadalafil in pentylenetetrazole induced convulsion model. Gülhane Tıp Derg 58:286–290
Sontag EM, Lotz GP, Agrawal N, Tran A, Aron R, Yang G et al (2012) Methylene blue modulates huntingtin aggregation intermediates and is protective in Huntington’s disease models. J Neurosci 32(32):11109–11119. https://doi.org/10.1523/JNEUROSCI.0895-12.2012
Stack C, Jainuddin S, Elipenahli C, Gerges M, Starkova N, Starkov AA, Jové M, Portero-Otin M, Launay N, Pujol A, Kaidery NA, Thomas B, Tampellini D, Beal MF, Dumont M (2014) Methylene blue upregulates Nrf2/ARE genes and prevents tau-related neurotoxicity. Hum Mol Genet 23(14):3716–3732. https://doi.org/10.1093/hmg/ddu080
Talley Watts L, Long JA, Chemello J, Van Koughnet S, Fernandez A, Huang S, Shen Q, Duong TQ (2014) Methylene blue is neuroprotective against mild traumatic brain injury. J Neurotrauma 1;31(11):1063–1071. https://doi.org/10.1089/neu.2013.3193
Turski L, Ikonomidou C, Turski WA, Bortolotto ZA, Cavalheiro EA (1989) Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse 3(2):154–171
Volke V, Wegener G, Vasar E, Rosenberg R (1999) Methylene blue inhibits hippocampal nitric oxide synthase activity in vivo. Brain Res 826(2):303–305
Walter-Sack I, Rengelshausen J, Oberwittler H, Burhenne J, Mueller O, Meissner P, Mikus G (2009) High absolute bioavailability of methylene blue given as an aqueous oral formulation. Eur J Clin Pharmacol 65(2):179–189. https://doi.org/10.1007/s00228-008-0563-x
Wesolowska A, Nikiforuk A, Chojnacka-Wójcik E (2006) Anticonvulsant effect of the selective 5-HT1B receptor agonist CP 94253 in mice. Eur J Pharmacol 541(1–2):57–63. https://doi.org/10.1016/j.ejphar.2006.04.049
Wiklund L, Basu S, Miclescu A, Wiklund P, Ronquist G, Sharma HS (2007) Neuro- and cardioprotective effects of blockade of nitric oxide action by administration of methylene blue. Ann N Y Acad Sci 1122:231–244. https://doi.org/10.1196/annals.1403.016
Wink DA, Mitchell JB (1998) Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 25(4–5):434–456
Wrubel KM, Riha PD, Maldonado MA, McCollum D, Gonzalez-Lima F (2007) The brain metabolic enhancer methylene blue improves discrimination learning in rats. Pharmacol Biochem Behav 86(4):712–717. https://doi.org/10.1016/j.pbb.2007.02.018
Zimmer LA, Ennis M, Wiley RG, Shipley MT (1998) Nerve gas-induced seizures: role of acetylcholine in the rapid induction of Fos and glial fibrillary acidic protein in piriform cortex. J Neurosci 18(10):3897–3908
Zulian GB, Tullen E, Maton B (1995) Methylene blue for ifosfamide-associated encephalopathy. N Engl J Med 332(18):1239–1240. https://doi.org/10.1056/NEJM199505043321817
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments were conducted in accordance with the ethical guidelines for care, use, and handling of laboratory animals by the Ethics Committee of the NRC and followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (publication no. 85-23, revised 1985).
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Salam, O.M.E., Sleem, A.A., Sayed, M.A.E.B.M. et al. Methylene blue protects against pentylenetetrazole-induced seizures, oxidative stress, and neuronal injury. Comp Clin Pathol 29, 341–354 (2020). https://doi.org/10.1007/s00580-019-03060-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-019-03060-4