Abstract
Purpose
Methylene blue (MB) has recently been reevaluated for malaria treatment. With the aim of excluding treatment failures due to low bioavailability, we have investigated the absolute bioavailability of MB given as an aqueous oral formulation and its interaction with chloroquine (CQ).
Methods
A phase I study in 16 healthy individuals was performed as a monocenter prospective open randomized intra-individual cross-over comparison of MB single doses [50 mg intravenous (i.v.), 500 mg orally, separated by a 1-week wash-out]. After a second week, the group was split for a randomized parallel group comparison of CQ 750 mg administered orally alone or combined with 500 mg MB orally.
Results
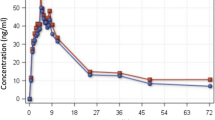
Mean MB plasma area under the substrate concentration–time curve (\({\text{AUC}}_{0 - \infty } \)) was 7,639 ± 3,384 ng/mL*h and 51,171 ± 17,147 ng/mL*h after i.v. and oral administration, respectively (dosage 1:10), and 76,897 ± 46,037 ng/mL*h after MB combined with CQ. The absolute bioavailability was 72.3 ± 23.9%. Co-administration with CQ significantly increased MB plasma concentrations (p ≤ 0.016); CQ kinetics remained unaffected.
Conclusion
The absolute bioavailability of MB is high. Co-administration of MB and CQ increases plasma, but not whole blood MB concentrations.




Similar content being viewed by others
References
Aeschlimann C, Kuepfer A, Schefer H, Cerny T (1998) Comparative pharmacokinetics of oral and intravenous ifosfamide/mesna/methylene blue therapy. Drug Metab Dispos 26:883–890
Akoachere M, Buchholz K, Fischer E, Burhenne J, Haefeli WE, Schirmer RH, Becker K (2005) In vitro assessment of methylene blue on chloroquine-sensitive and -resistant Plasmodium falciparum strains reveals synergistic action with artemisinins. Antimicrob Agents Chemother 49:4592–4597
Burhenne J, Riedel K-D, Rengelshausen J, Meissner P, Mueller O, Mikus G, Haefeli WE, Walter-Sack I (2008) Quantification of cationic anti-malaria agent methylene blue in different human biological matrices using cation exchange chromatography coupled to tandem mass spectrometry. J Chromatogr B 863:273–282
DiSanto AR, Wagner JG (1972) Pharmacokinetics of highly ionized drugs II: methylene blue—absorption, metabolism, and excretion in man and dog after oral administration. J Pharm Sci 61:1086–1090
Donati A, Conti G, Loggi S, Muench C, Coltrinari R, Pelaia P, Pietropaoli P, Preiser J-C (2002) Does methylene blue administration to septic shock patients affect vascular permeability and blood volume. Crit Care Med 30:2271–2277
Ducharme J, Farinotti R (1996) Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin Pharmacokinet 31:257–274
Ehrlich P (1913) Chemotherapeutics: scientific principles, methods, and results. Lancet 182:445–451
European Medicines Agency (EMEA) Committee for Medicinal Products for Human Use (2006) Reflection paper: Formulations of choice for the pediatric population. Available at: http://www.emea.europa.eu/pdfs/human/paediatrics/19481005en.pdf. Accessed 8 July 2008
Evora PR, Simon MR (2007) Role of nitric oxide production in anaphylaxis and its relevance for the treatment of anaphylactic hypotension with methylene blue. Ann Allergy Asthma Immunology 99:306–313
Ferreira C (1893) Sur l`emploi du bleu de méthylène dans la malaria infantile. Rev Ther Med Chir 488–525
Frisk-Holmberg M, Bergqvist Y, Termond E, Domeij-Nyberg B (1984) The single dose kinetics of chloroquine and its major metabolite desethylchloroquine in healthy subjects. Eur J Clin Pharmacol 26:521–530
Gustafsson LL, Walker O, Alván G, Beermann B, Estevez F, Gleisner L, Lindstroem B, Sjoeqvist F (1983) Disposition of chloroquine in man after single intravenous and oral doses. Br J Clin Pharmacol 15:471–479
Gut F, Schiek W, Haefeli WE, Walter-Sack I, Burhenne J (2008) Cation exchange resins as pharmaceutical carriers for methylene blue: Binding and release. Eur J Pharm Biopharm 69:582–587
Guttmann P, Ehrlich P (1891) Ueber die Wirkung des Methylenblau bei Malaria. Berlin Klin Wochenschr 28:953–956
Khan MA, North AP, Chadwick DR (2007) Prolonged postoperative altered mental status after methylene blue infusion during parathyroidectomy: a case report and review of the literature. Ann R Coll Surg Eng 89:W9–W11
Kuepfer A, Aeschlimann C, Wermuth B, Cerny T (1994) Prophylaxis and reversal of ifosfamide encephalopathy with methylene blue. Lancet 343:763–764
Mandi G, Witte S, Meissner P, Coulibaly B, Mansmann U, Rengelshausen J, Schiek W, Jahn A, Sanon M, Wuest K, Walter-Sack I, Mikus G, Burhenne J, Riedel K-D, Schirmer H, Kouyaté B, Mueller O (2005) Safety of the combination of chloroquine and methylene blue in healthy adult men with G6PD deficiency from rural Burkina Faso. Trop Med Int Health 10:32–38
Meissner P, Mandi G, Witte S, Coulibaly B, Mansmann U, Rengelshausen J, Schiek W, Jahn A, Sanon M, Tapsoba T, Walter-Sack I, Mikus G, Burhenne J, Riedel K-D, Schirmer H, Kouyaté B, Mueller O (2005) Safety of the combination of chloroquine and methylene blue in the treatment of uncomplicated falciparum malaria in young children of Burkina Faso. Malaria J 4:45
Meissner PE, Mandi G, Coulibaly B, Witte S, Tapsoba T, Mansmann U, Rengelshausen J, Schiek W, Jahn A, Walter-Sack I, Mikus G, Burhenne J, Riedel K-D, Schirmer RH, Kouyate B, Mueller O (2006) Methylene blue for malaria in Africa: results from a dose-finding study in combination with chloroquine. Malaria J 5:84
Minzi OM, Rais M, Svensson JO, Gustafsson LL, Ericsson O (2003) High-performance chromatographic method for determination of amodiaquine, chloroquine and their monodesethyl metabolites in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci 783:473–480
Neto AMO, Duarte NM, Vicente WVA, Viaro F, Evora PRB (2003) Methylene blue: an effective treatment for contrast medium-induced anaphylaxis. Med Sci Monit 9:CS102–CS106
Noedl H, Chanthap L, Se Y, Socheat D, Peou S, Schaechter K, Srivichai S, Teja-Isavadharm P, Smith B, Jongsakul K, Surasri S, Fukuda M (2007) Artemisinin resistance in Cambodia. Oral presentation, 5th Eur Congress Trop Med Int Health. Amsterdam
Pelgrims J, De Vos F, Van den Brande J, Schrijvers D, Prové A, Vermorken JB (2000) Methylene blue in the treatment and prevention of ifosfamide-induced encephalopathy: report of 12 cases and review of the literature. Br J Cancer 82:291–294
Peter C, Hongwan D, Kuepfer A, Lauterburg BH (2000) Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur J Clin Pharmacol 56:247–250
Politis C, Kavallierou L, Hantziara S, Katsea P, Triantaphylou V, Richardson C, Tsoutsos D, Anagnostopoulos N, Gorgolidis G, Ziroyannis P (2007) Quality and safety of fresh-frozen plasma inactivated and leuco-reduced with the Theraflex methylene blue system including the Blueflex filter: 5 years’ experience. Vox Sang 92:319–326
Rengelshausen J, Burhenne J, Froehlich M, Tayrouz Y, Singh SK, Riedel K-D, Mueller O, Hoppe-Tichy T, Haefeli WE, Mikus G, Walter-Sack I (2004) Pharmacokinetic interaction of chloroquine and methylene blue combination against malaria. Eur J Clin Pharmacol 60:709–715
Schirmer RH, Coulibaly B, Stich A, Scheiwein M, Merkle H, Eubel J, Becker K, Becher H, Mueller O, Zich T, Schiek W, Kouyaté B (2003) Methylene blue as an antimalarial agent. Redox Rep 8:272–275
Simonsen AC, Sørensen H (1999) Clinical tolerance of methylene blue virus-inactivated plasma. Vox Sang 77:210–217
Stevens S, Werner L, Mamalis N (2007) Corneal edema and permanent blue discoloration of a silicone intraocular lens by methylene blue. Ophthalmic Surg Lasers Imaging 38:136–141
Williamson LM, Cardigan R, Prowse CV (2003) Methylene blue-treated fresh-frozen plasma: what is its contribution to blood safety. Transfusion 43:1322–1329
Zoungrana A, Coulibaly B, Sié A, Walter-Sack I, Mockenhaupt FP, Kouyaté B, Schirmer RH, Klose C, Mansmann U, Meissner P, Mueller O (2008) Safety and efficacy of methylene blue combined with artesunate or amodiaquine for uncomplicated falciparum malaria: a randomized controlled trial from Burkina Faso. Available at: http://www.plosone.org/3:e1630
Zulian GB, Tullen E, Maton B (1995) Methylene blue for ifosfamide-associated encephalopathy. N Engl J Med 332:1239–1240
Acknowledgements
The study was funded by an award from DSM Fine Chemicals Austria, Linz, Austria and by the Deutsche Forschungsgemeinschaft (SFB 544 “Control of Tropical Infectious Diseases” at the Ruprecht-Karls-University Heidelberg). We thank Mrs. Monika Maurer and Mrs. Brigitte Tayrouz for their excellent technical assistance.
Financial disclosure
None of the authors has financial or other relationships that are relevant to the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walter-Sack, I., Rengelshausen, J., Oberwittler, H. et al. High absolute bioavailability of methylene blue given as an aqueous oral formulation. Eur J Clin Pharmacol 65, 179–189 (2009). https://doi.org/10.1007/s00228-008-0563-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0563-x