Abstract
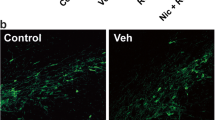
We investigated the effect of a single injection of rotenone into the striatum on the development of oxidative stress, nigrostriatal cell injury and motor alterations in rats. Rotenone (1, 3, 5 and 9 mM; 5 μL/rat) or the vehicle (dimethyl sulfoxide) was injected into the right striatum. Control rats received the vehicle only. Rats were allowed to recover from the operation and were tested for behavioural changes on 7th and 30th days after rotenone injection. Biochemical markers of oxidative stress including malondialdehyde (MDA), reduced glutathione (GSH), nitric oxide, paraoxonase 1 (PON1) activity and Q10 enzyme as well as monoamine neurotransmitters in the brain were determined after 30 days of rotenone treatment. Histopathology and tyrosine hydroxylase immunohistochemistry were also performed. Results: Intrastriatal injection of rotenone at 9 mM caused immediate death of rats. No mortality was observed with the lower concentrations of the pesticide. Rotenone at 1–5 mM resulted in increased brain oxidative stress in a dose-dependent manner. MDA increased by 23.5–64.9 %, while GSH decreased by 20.4–24.1 % in the contralateral cerebral hemisphere. Nitric oxide increased by 20.2–41.7 % in ipsilateral cortex. PON1 activity decreased by 12.5–38.2 % in ipsilateral cerebral cortex and by 31.2–65.3 % in ipsilateral striatum, respectively, but coenzyme Q10 increased in the ipsilateral cortex by 21–26.3 %. There was decreased dopamine and serotonin in the ipsilateral striatum after rotenone injection. Tyrosine hydroxylase immunoreactivity was markedly decreased in ipsilateral substantia nigra in the rotenone-treated in contrast to the vehicle-treated rats. Rotenone increased the number of degenerated cells in substantia nigra in a dose-dependent manner. It also caused depletion of pigment granules from cells. Degenerative changes were also observed in the contralateral hippocampus and cortex especially after the highest dose of rotenone. The number of spontaneous rears made during 30 min in the cylinder was decreased in both limbs; the decrease being more evident in the ipsilateral side. Thus, a single intrastriatal injection of rotenone (a) caused a significant nigrostriatal degeneration and loss of dopamine and serotonin from the striatum; (b) elicited cell degeneration in the hippocampus and cortex; (c) induced oxidative stress and neuronal injury (this latter effect of rotenone was not region specific); and (d) the motor deficits (decreased rearing activity) occurred in both limbs.






Similar content being viewed by others
References
Alam M, Schmidt WJ (2002) Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res 136:317–324
Berardelli A, Rothwell JC, Thompson PD, Hallett M (2001) Pathophysiology of bradykinesia in Parkinson’s disease. Brain 124:2131–2146
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3:1301–1306
Clifford W, Shults T (2005) Therapeutic role of coenzyme Q10 in Parkinson’s disease. Pharmacol Ther 107:120–130
Crane FL (2001) Biochemical functions of coenzyme Q10. J Am Coll Nutr 20:591–598
Delaville C, Deurwaerdère PD, Benazzouz A (2011) Noradrenaline and Parkinson’s disease. Front Syst Neurosci 5:31
Double KL, Gerlach M, Youdim MB, Riederer P (2000) Impaired iron homeostasis in Parkinson’s disease. J Neural Transm Suppl 60:37–58
Dringen R (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62:649–671
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem 82:70–77
Feng Y, Liang ZH, Wang T, Qiao X, Liu HJ, Sun SG (2006) alpha-Synuclein redistributed and aggregated in rotenone-induced Parkinson’s disease rats. Neurosci Bull 22:288–293
Ferrante RJ, Schulz JB, Kowall NW, Beal MF (1997) Systemic administration of rotenone produces selective damage in the striatum and globus pallidus, but not in the substantia nigra. Brain Res 753:157–162
Furlong CE (2008) Paraoxonases: an historical perspective. In: Mackness B, Mackness M, Aviram M, Paragh G (eds) The paraoxonases: their role in disease development and xenobiotic metabolism. Springer, Dordrecht, pp 3–31
Halliwell B (1992) Reactive oxygen species and the central nervous system. J Neurochem 59:1609–1623
Halliwell N (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18:685–716
Halliwell B, Cross CE (1994) Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect 102(Suppl 10):5–12
Higashino K, Takahashi Y, Yamamura Y (1972) Release of phenyl acetate esterase from liver microsomes by carbon tetrachloride. Clin Chim Acta 41:313–320
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397
Horvath TL, Diano S, Leranth C, Garcia-Segura LM, Cowley MA, Shanabrough M, Elsworth JD, Sotonyi P, Roth RH, Dietrich EH, Matthews RT, Barnstable CJ, Redmond DE Jr (2003) Coenzyme Q induces nigral mitochondrial uncoupling and prevents dopamine cell loss in a primate model of Parkinson’s disease. Endocrinology 144:2757–2760
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Islam MB (2006) Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol 51:45–66
Jamroz-Wisniewska A, Beltowski J, Stelmasiak Z, Bartosik-Psujek H (2009) Paraoxonase 1 activity in different types of multiple sclerosis. Mult Scler 15:399–402
Janáky R, Cruz–Aguado R, Oja SS, Shaw CA (2007) Glutathione in the nervous system: roles in neural function and health and implications for neurological disease. In: Lajtha A, Oja SS, Schousboe A, Saransaari P (eds) Handbook of neurochemistry and molecular neurobiology. Springer-Verlag, Berlin, pp 349–385
Jellinger KA (2002) Recent developments in the pathology of Parkinson’s disease. J Neural Transm Suppl 62:347–376
Kalia LV, Brotchie JM, Fox SH (2013) Novel nondopaminergic targets for motor features of Parkinson’s disease: review of recent trials. Mov Disord 28:131–144
La Du BN (1992) Human serum paraoxonase: arylesterase. In: Kalow W (ed) Pharmacogenetics of drug metabolism. Pergamon, Elmford, pp 51–91
Lapointe N, St-Hilaire M, Martinoli MG, Blanchet J, Gould P, Rouillard C, Cicchetti F (2004) Rotenone induces non-specific central nervous system and systemic toxicity. FASEB J 18:717–719
Lotharius J, Brundin P (2002) Pathogenesis of Parkinson’s disease: dopamine, vesicles and α-synuclein. Nat Rev Neurosci 3:932–942
Mischley LK, Allen J, Bradley R (2012) PD patients had a significantly greater odds of deficiency in coenzyme Q10 status. Coenzyme Q10 deficiency in patients with Parkinson’s disease. J Neurol Sci 318:72–75
Moshage H, Kok B, Huizenga JR (1995) Nitrite and nitrate determination in plasma: a critical evaluation. Clin Chem 41:892–896
Mulcahy P, Walsh S, Paucard A, Rea K, Dowd E (2011) Characterisation of a novel model of Parkinson’s disease by intra-striatal infusion of the pesticide rotenone. Neuroscience 181:234–242
Olanow CW, Tatton WG (1999) Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci 22:123–144
Pankratz N, Foroud T (2007) Genetics of Parkinson disease. Genet Med 9:801–811
Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, Greenamyre JT (2005) Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem 280:42026–42035
Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS (2001) Environmental risk factors and Parkinson’s disease: a meta-analysis. Environ Res 86:122–127
Ravenstijn PG, Merlini M, Hameetman M, Murray TK, Ward MA, Lewis H, Ball G, Mottart C, de Ville Goyet C, Lemarchand T, van Belle K, O’Neill MJ, Danhof M, de Lange EC (2008) The exploration of rotenone as a toxin for inducing Parkinson’s disease in rats, for application in BBB transport and PK-PD experiments. J Pharmacol Toxicol Methods 57:114–130
Ren Y, Feng J (2007) Rotenone selectively kills serotonergic neurons through a microtubule-dependent mechanism. J Neurochem 103:303–311
Rodrigo L, Hernández AF, López-Caballero JJ, Gil F, Pla A (2001) Immunohistochemical evidence for the expression and induction of paraoxonase in rat liver, kidney, lung and brain tissue. Implications for its physiological role. Chem Biol Interact 137:123–137
Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, de Groot H (1994) Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids 59:383–388
Santens P, Boon P, Van Roost D, Caemaert J (2003) The pathophysiology of motor symptoms in Parkinson’s disease. Acta Neurol Belg 103:129–134
Sayre LM, Perry G, Smith MA (2008) Oxidative stress and neurotoxicity. Chem Res Toxicol 21:172–188
Schmidt WJ, Alam M (2006) Controversies on new animal models of Parkinson’s disease pro and con: the rotenone model of Parkinson’s disease (PD). J Neural Transm Suppl 70:273–376
Schulz JB, Lindenau J, Seyfried J, Dichgans J (2000) Glutathione, oxidative stress and neurodegeneration. Eur J Biochem 267:4904–1143
Sechi G, Deledda MG, Bua G, Satta WM, Deiana GA, Pes GM, Rosati G (1996) Reduced intravenous glutathione in the treatment of early Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry 20:1159–1170
Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT (2003a) Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci 23:10756–10764
Sherer TB, Kim JH, Betarbet R, Greenamyre JT (2003b) Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol 179:9–16
Shults CW, Oakes D, Kieburtz K, Beal MF, Haas R, Plumb S, Juncos JL, Nutt J, Shoulson I, Carter J, Kompoliti K, Perlmutter JS, Reich S, Stern M, Watts RL, Kurlan R, Molho E, Harrison M, Lew M, Parkinson Study Group (2002) Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol 59:1541–1550
Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD (1994) Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol 36:348–355
Sohmiya M, Tanaka M, Tak NW, Yanagisawa M, Tanino Y, Suzuki Y, Okamoto K, Yamamoto Y (2004) Redox status of plasma coenzyme Q10 indicates elevated systemic oxidative stress in Parkinson’s disease. J Neurol Sci 223:161–166
Swarnkar S, Singh S, Sharma S, Mathur R, Patro IK, Nath C (2011) Rotenone induced neurotoxicity in rat brain areas: a histopathological study. Neurosci Lett 501:123–127
Thiffault C, Langston JW, Di Monte DA (2000) Increased striatal dopamine turnover following acute administration of rotenone to mice. Brain Res 885:283–288
Townsend DM, Tew KD, Tapiero H (2003) The importance of glutathione in human disease. Biomed Pharmacother 57:145–155
Uversky VN (2004) Neurotoxicant-induced animal models of Parkinson’s disease: understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res 318:225–241
Vanacore N, Nappo A, Gentile M, Brustolin A, Palange S, Liberati A, Di Rezzel S, Caldoral G, Gasparinil M, Benedetti F, Bonifatil V, Forastiere F, Quercia A, Mecol G (2002) Evaluation of risk of Parkinson’s disease in a cohort of licensed pesticide users. Neurol Sci 23(suppl. 2):S119–S120
Wang W, Ballatori N (1998) Endogenous glutathione conjugates: occurrence and biological functions. Pharmacol Rev 50:335–356
Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM, Navab M (1995) Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest 96:2882–2891
Wehr H, Bednarska-Makaruk M, Graban A, Lipczyńska-Łojkowska W, Rodo M, Bochyńska A, Ryglewicz D (2009) Paraoxonase activity and dementia. J NeurolSci 283:107–108
Wei T, Chen C, Hou J, Xin W, Mori A (2000) Nitric oxide induces oxidative stress and apoptosis in neuronal cells. Biochim Biophys Acta 1498:72–79
Xiong N, Huang J, Zhang Z, Zhang Z, Xiong J, Liu X, Jia M, Wang F, Chen C, Cao X, Liang Z, Sun S, Lin Z, Wang T (2009) Stereotaxical infusion of rotenone: a reliable rodent model for Parkinson’s disease. PLoS One 4:e7878
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Salam, O.M.E., Khadrawy, Y.A., Youness, E.R. et al. Effect of a single intrastriatal rotenone injection on oxidative stress and neurodegeneration in the rat brain. Comp Clin Pathol 23, 1457–1467 (2014). https://doi.org/10.1007/s00580-013-1807-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-013-1807-4