Abstract
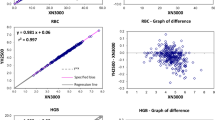
The ProCyte Dx™ was introduced as an in-house hematology analyzer based on focused flow impedance and flow cytometry. It provides a complete hemogram including a five-part leukocyte differential and reticulocyte count. The aim of the study was to evaluate the ProCyte Dx for dogs and cats. EDTA-anticoagulated blood samples from healthy or diseased dogs (n = 270) and cats (n = 176) were analyzed within 3 to 6 h of sampling. Routine hemogram variables including reticulocytes were compared with reference methods, i.e., the ADVIA 2120, a 200-cell manual differential leukocyte count, and manual reticulocyte counts. Data were analyzed twice (prior to and after dot plot analysis, with the exclusion of samples with invalid separations of cellular populations). Coefficients of variation were <3 % for complete blood cell count and <7 % for differential count, except for eosinophils (cat, 17 %), lymphocytes (cat, 30 %), platelet counts (PLTs; dog, 14 %), and reticulocytes (dog and cat, 16 and 22 %, respectively). Spearman’s rank correlation coefficients (r s) revealed a good to excellent (r s = 0.99–0.80) correlation between both analyzers, except for the mean corpuscular hemoglobin concentration (MCHC; r s = 0.56–0.44), cat reticulocytes (r s = 0.77), and differential count prior to dot plot analysis. Biases were generally close to 0; however, large biases were seen for hemoglobin (HGB), mean corpuscular hemoglobin, MCHC, mean corpuscular volume, PLTs, and differential count prior to dot plot analysis. The majority of variables correlated favorably with the ADVIA 2120. The large biases of HGB and HGB-derived variables were due to the methodology of the ADVIA. Dot plot analysis is an additional tool for quality assurance, and a manual differential count is recommended in case of invalid separation of cellular populations.





Similar content being viewed by others
Notes
IDEXX Laboratories, Westbrook, ME, USA.
Abbott Laboratories, Abbott Park, IL, USA.
Siemens Medical Solution Diagnostics, Eschborn, Germany.
IDEXX ProCyte Dx Stain Pack.
Analyze-it Software Ltd., Leeds, UK.
GraphPad Software Inc., La Jolla, CA, USA.
Abbreviations
- CBC:
-
Complete blood cell count
- FSC:
-
Forward scatter
- EOS:
-
Eosinophils
- HGB:
-
Hemoglobin
- HCT:
-
Hematocrit value
- MCH:
-
Mean corpuscular hemoglobin
- MCV:
-
Mean corpuscular volume
- LYMPH:
-
Lymphocytes
- MCHC:
-
Mean corpuscular hemoglobin concentration
- MONO:
-
Monocytes
- NEUT:
-
Neutrophils
- PCV:
-
Packed cellular volume
- PLT-I:
-
Platelets measured with impedance method
- PLT-O:
-
Platelets measured with optical method
- RBC:
-
Red blood cells
- r s :
-
Spearman’s rho
- SSC:
-
Side scatter
- SFL:
-
Side fluorescence
- WBC:
-
White blood cells
References
Anonymous (2008) Customers information. Software-info XT-IV series software version 00-09, 1st edn.
Bauer N, Moritz A (2008) Evaluation of three methods for measurement of hemoglobin and calculated hemoglobin parameters with the ADVIA 2120 and ADVIA 120 in dogs, cats, and horses. Vet Clin Pathol 37:173–179
Bauer N, Nakagawa J, Dunker C, Failing K, Moritz A (2011a) Evaluation of the automated hematology analyzer Sysmex XT-2000iV™ compared to the ADVIA® 2120 for its use in dogs, cats, and horses. Part I—precision, linearity, and accuracy of complete blood cell count. J Vet Diagn Investig 23:1168–1180. doi:10.1177/1040638711425572
Bauer N, Nakagawa J, Dunker C, Failing K, Moritz A (2011b) Evaluation of the automated hematology analyzer Sysmex XT-2000iV™ compared to the ADVIA 2120® for its use in dogs, cats, and horses. Part II—accuracy of leukocyte differential and reticulocyte count, impact of anticoagulant and sample aging. J Vet Diagn Investig 24:74–89
Boes K (2010) Validation of the ProCyte Dx Hematology Analyzer for Canine Complete Blood Counts. CPB 69700 Research Seminar
Bollinger PB, Drewinko B, Brailas CD, Smeeton NA, Trujillo JM (1987) The technicon H*1—an automated hematology analyzer for today and tomorrow. Complete blood count parameters. Am J Clin Pathol 87:71–78
Furlanello T, Tasca S, Caldin M et al (2006) Artifactual changes in canine blood following storage, detected using the ADVIA 120 hematology analyzer. Vet Clin Pathol 35:42–46
ICHS (1993) Recommendations of the International Council for Standardization in Haematology for ethylenediaminetetraacetic acid anticoagulation of blood for blood cell counting and sizing. International Council for Standardization in Haematology: expert panel on cytometry. Am J Clin Pathol 100:371
ICHS (1994) Guidelines for the evaluation of blood cell analysers including those used for differential leucocyte and reticulocyte counting and cell marker applications. International Council for Standardization in Haematology: prepared by the ICSH Expert Panel on Cytometry. Clin Lab Haematol 16:157–174
Koepke JA, Bentley SA, Pierre RV et al (1992) Reference leukocyte differential count (proportional) and evaluation of instrumental methods. A reference method of the evaluation of automated differential counters, based on the visual differential count. NCCLS Document H-20-A, vol. 27, no. 4
Lilliehook I, Tvedten H (2009a) Validation of the Sysmex XT-2000iV hematology system for dogs, cats, and horses. I. Erythrocytes, platelets, and total leukocyte counts. Vet Clin Pathol 38:163–174
Lilliehook I, Tvedten H (2009b) Validation of the Sysmex XT-2000iV hematology system for dogs, cats, and horses. II. Differential leukocyte counts. Vet Clin Pathol 38:175–182
Lilliehook I, Tvedten H (2011) Errors in basophil enumeration with 3 veterinary hematology systems and observations on occurrence of basophils in dogs. Vet Clin Pathol 40:450–458. doi:10.1111/j.1939-165X.2011.00353.x
Mathers RA, Evans GO, Bleby J, Tornow T (2008) Evaluation of the Sysmex XT-2000iV haematology analyser for rat, dog and mouse whole blood samples. Comp Clin Pathol 17:137–144
Moritz A (2001) Der Einsatz lasergestützer Multiparameter Hämatologiesysteme in der Veterinärmedizin (The use of automated laser-based multiparameter hematology systems in veterinary medicine). 1 ed Büchse der Pandora,
Moritz A, Becker M (2010) Automated hematology systems. In: Weiss DJ, Wardrop KJ (eds) Schalm's veterinary hematology. Wiley-Blackwell, Ames
Moritz A, Fickenscher Y, Meyer K et al (2004) Canine and feline hematology reference values for the ADVIA 120 hematology system. Vet Clin Pathol 133:32–38
Moritz A, Walcheck BK, Weiss DJ (2005) Evaluation of flow cytometric and automated methods for detection of activated platelets in dogs with inflammatory disease. Am J Vet Res 66:325–329
Nakagawa J (2011) Evaluation der Hämatologiesysteme Sysmex pocH-100iV Diff und XT-2000iV für die Tierart Katze [Evaluation of the hematology analyzer Sysmex pocH-100iV Diff and XT-2000iV for cats]. Thesis work, Thesis Giessen
Neuerer FF (2005) Evaluation des vollautomatischen Hämatologiegerätes CELL-DYN 3500 im klinischen Einsatz bei Hund und Katze [Evaluation of the automated hematology analyzer CELL-DYN 3500 for the clinical use in dog and cat]. Thesis work Munich
Papasouliotis K, Cue S, Crawford E, Pinches M, Dumont M, Burley K (2008) Comparison of white blood cell differential percentages determined by the in-house LaserCyte hematology analyzer and a manual method. Vet Clin Pathol 35:295–302
Petrie A, Watson P (1999) Statistics for veterinary and animal science. Blackwell, Oxford
Reed GF, Lynn F, Meade BD (2002) Use of coefficient of variation in assessing variability of quantitative assays. Clin Diagn Lab Immunol 9:1235–1239
Rizzi TE, Meinkoth JH, Clinkenbeard KD (2010) Normal hematology of the dog. In: Weiss DJ, Wardrop KJ (eds) Schalm's veterinary hematology. Blackwell, Ames, pp 799–810
Rumke CL (1960) Laboratory aids. Variability of results in differential cell counts on blood smears. Triangle 4:154–158
Tvedten H, Moritz A (2010) Reticulocyte and Heinz body staining and enumeration. In: Weiss DJ, Wardrop KJ (eds) Schalm's veterinary hematology. Wiley-Blackwell, Ames
Westgard JO (2003) Basic method validation. Westgard Quality Corporation, Milwaukee
Conflict of interest
The authors state that there is no conflict of interest for any of the authors of this publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goldmann, F., Bauer, N. & Moritz, A. Evaluation of the IDEXX ProCyte Dx analyzer for dogs and cats compared to the Siemens ADVIA 2120 and manual differential. Comp Clin Pathol 23, 283–296 (2014). https://doi.org/10.1007/s00580-012-1608-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-012-1608-1