Abstract
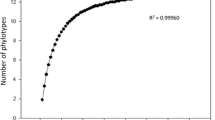
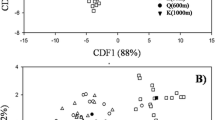
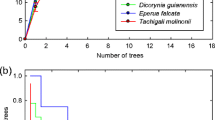
As it is well known, arbuscular mycorrhizal (AM) colonization can be initiated from the following three types of fungal propagules: spores, extraradical mycelium (ERM), and mycorrhizal root fragments harboring intraradical fungal structures. It has been shown that biomass allocation of AM fungi (AMF) among these three propagule types varies between fungal taxa, as also differs the ability of the different AMF propagule fractions to initiate new colonizations. In this study, the composition of the AMF community in the roots of rosemary (Rosmarinus officinalis L., a characteristic Mediterranean shrub), inoculated with the three different propagule types, was analyzed. Accordingly, cuttings from this species were inoculated with either AMF spores, ERM, or colonized roots extracted from a natural soil. The AMF diversity within the rosemary roots was characterized using terminal restriction fragment length polymorphism (T-RFLP) of the small subunit (SSU) rDNA region. The AMF community established in the rosemary plants was significantly different according to the type of propagule used as inoculum. AMF taxa differed in their ability to initiate new colonizations from each propagule type. Results suggest different colonization strategies for the different AMF families involved, Glomeraceae and Claroideoglomeraceae colonizing mainly from colonized roots whereas Pacisporaceae and Diversisporaceae from spores and ERM. This supports that AMF taxa show contrasting life-history strategies in terms of their ability to initiate new colonizations from the different propagule types. Further research to fully understand the colonization and dispersal abilities of AMF is essential for their rational use in ecosystem restoration programs.

Similar content being viewed by others
References
Abbott LK, Robson AD, Gazey C (1994) Selection of inoculants vesicular-arbuscular mycorrhizal fungi. In: Norris JR, Read D, Varma AK (eds) Methods in microbiology, vol. 24. Techniques for the study of mycorrhiza. Academic, London, pp 1–21
Allen MF, Kitajima K (2013) In situ high-frequency observations of mycorrhizas. New Phytol 200:222–228
Barea JM, Pozo MJ, López-Ráez JA, Aroca R, Ruíz-Lozano JM, Ferrol N, Azcón R, Azcón-Aguilar C (2013) Mycorrhizas and their significance in promoting soil-plant systems sustainability against environmental stresses. In: Rodelas B, González-López J (eds) Beneficial plant-microbial interactions: ecology and applications. CRC Press, USA, pp 353–387
Biermann B, Linderman RG (1983) Mycorrhizal roots, intrarradical vesicles and extraradical vesicles as inoculum. New Phytol 95:97–105
Brundrett MC, Abbot LK, Jasper DA (1999) Glomalean fungi from tropical Australia. I. Comparison of the effectiveness of isolation procedures. Mycorrhiza 8:305–314
Chagnon PL (2014) Ecological and evolutionary implications of hyphal anastomosis in arbuscular mycorrhizal fungi. FEMS Microbiol Ecol 88:437–444
Chagnon PL, Bradley RL, Maherali H, Klironomos JN (2013) A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci 18:484–491
Chytrý M, Tichý L, Holt J, Botta-Dukát Z (2002) Determination of diagnostic species with statistical fidelity measures. J Veg Sci 13:79–90
de Cáceres M, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574
de la Providencia IE, de Souza FA, Fernandez F, Delmas NS, Declerck S (2005) Arbuscular mycorrhizal fungi reveal distinct patterns of anastomosis formation and hyphal healing mechanisms between different phylogenic groups. New Phytol 165:261–271
Dickie IA, FitzJohn RG (2007) Using terminal restriction fragment length polymorphism (T-RFLP) to identify mycorrhizal fungi: a methods review. Mycorrhiza 17:259–270
Fitter AH (2005) Darkness visible: reflections on underground ecology. J Ecol 93:231–243
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from the soil by wet sieving and decanting. Trans Brit Mycol Soc 46:235–244
Grime JP, Mackey JM, Hillier SH, Read DJ (1987) Floristic diversity in a model system using experimental microcosms. Nature 328:420–422
Gutjahr C, Parniske M (2013) Cell and developmental biology of arbuscular mycorrhiza symbiosis. Ann Rev Cell Dev Biol 29:593–617
Hart MM, Reader JR (2002) Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol 153:335–344
Helgason T, Fitter AH (2009) Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (Phylum Glomeromycota). J Exp Bot 60:2465–2480
Hempel S, Renker C, Buscot F (2007) Differences in the species composition of arbuscular mycorrhizal fungi in spore, root and soil communities in a grassland ecosystem. Environ Microbiol 9:1930–1938
Hewitt EJ (1952) Sand and water culture methods used in the study of plant nutrition. Technical Communication 22, Farnham Royal, Commonwealth Agricultural Bureaux, Bucks, London
Ijdo M, Schtickzelle N, Cranenbrouck S, Declerck S (2010) Do arbuscular mycorrhizal fungi with contrasting life-history strategies differ in their responses to repeated defoliation? FEMS Microbiol Ecol 72:114–122
Jakobsen I (2004) Hyphal fusion to plant species connections—giant mycelia and community nutrient flow. New Phytol 164:4–7
Jeffries P, Barea JM (2012) Arbuscular mycorrhiza—a key component of sustainable plant-soil ecosystems. In: Hock B (ed) The mycota IX. Springer, Berlín, pp 51–75
Klironomos JN, Hart MM (2002) Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 12:181–184
Lavorel S, Grigulis K, Lamarque P, Colace MP, Garden D et al (2011) Using plant functional traits to understand the landscape distribution of multiple ecosystem services. J Ecol 99:135–147
Lee J, Lee S, Young JPW (2008) Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol Ecol 65:339–349
López-García A, Azcón-Aguilar C, Barea JM (2014a) The interactions between plant life form and fungal traits of arbuscular mycorrhizal fungi determine the symbiotic community. Oecologia 176:1075–1086
López-García A, Palenzuela J, Barea JM, Azcón-Aguilar C (2014b) Life-history strategies of arbuscular mycorrhizal fungi determine succession into roots of Rosmarinus officinalis L., a characteristic woody perennial plant species from Mediterranean ecosystems. Plant Soil 379:247–260
Mäder P, Vierheilig H, Streitwolf-Engel R, Boller T, Frey B, Christine P, Wiemken A (2000) Transport of 15N from a soil compartment separated by a polytetrafluoroethylene membrane to plant roots via the hyphae of arbuscular mycorrhizal fungi. New Phytol 146:155–161
Maherali H, Klironomos JN (2007) Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316:1746–1748
Maherali H, Klironomos JN (2012) Phylogenetic and trait-based assembly of arbuscular mycorrhizal fungal communities. PLoS ONE 7, e36695
Martiny JBH, Bohannan BJ, Brown JH, Colwell RK, Fuhrman JA et al (2006) Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol 4:102–112
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82:290–297
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Miller RM, Jastrow JD (2000) Mycorrhizal fungi influence soil structure. In: Kapulnik Y, Douds DD (eds) Arbuscular mycorrhizae: molecular biology and physiology. Kluwer Academic Press, Dordrecht
Newsham KK, Fitter AH, Watkinson AR (1995) Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol Evol 10:407–411
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, et al. (2015) Vegan: community ecology package, ver.2.3-1. Available from http://CRAN.Rproject.org/package=vegan
Öpik M, Vanatoa A, Vanatoa E, Moora M, Davison J, Kalwij JM, Reier U, Zobel M (2010) The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol 188:223–241
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Brit Mycol Soc 55:158–161
Powell JR, Parrent JL, Hart MM, Klironomos JN, Rillig MC, Maherali H (2009) Phylogenetic trait conservatism and the evolution of functional trade-offs in arbuscular mycorrhizal fungi. Proc Roy Soc B-Biol Sci 276:4237–4245
Redecker D, Schüßler A, Stockinger H, Stürmer SL, Morton JB, Walker C (2013) An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 23:515–531
Ripley B, Venables B, Bates DM, Hornik K, Gebhardt A, Firth D (2014) MASS: support functions and datasets for Venables and Ripley’s MASS, ver. 7.3-33. [WWW document]. URL http://CRAN.Rproject.org/package=MASS
Saks Ü, Davison J, Öpik M, Vasar M, Moora M, Zobel M (2014) Root-colonizing and soil-borne communities of arbuscular mycorrhizal fungi in a temperate forest understorey. Botany 92:277–285
Schalamuk S, Cabello M (2010) Arbuscular mycorrhizal fungal propagules from tillage and no-tillage systems: possible effects on Glomeromycota diversity. Mycologia 102:261–268
Sieverding E (1991) Vesicular-arbuscular mycorrhyza management in tropical agrosystems. GTZ, Friedland
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, thirdth edn. Elsevier, Academic Press, New York
Smith SE, Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol 62:227–250
Tichy L, Chytry M (2006) Statistical determination of diagnostic species for site groups of unequal size. J Veg Sci 17:809–818
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R et al (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72
van der Heijden MGA, Martin FM, Selosse MA et al (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205:1406–1423
Varela-Cervero S, Vasar M, Davison J, Barea JM, Öpik M, Azcón-Aguilar C (2015) The composition of arbuscular mycorrhizal fungal communities differs among the roots, spores and extraradical mycelia associated with five Mediterranean plant species. Environ Microbiol 17:2882–2895
Voets L, de La Providencia IE, Declerck S (2006) Glomeraceae and Gigasporaceae differ in their ability to form hyphal networks. New Phytol 172:185–188
Werner G, Kiers ET (2015) Order of arrival structures arbuscular mycorrhizal colonization of plants. New Phytol 205:1515–1524
Acknowledgments
Sara Varela-Cervero thanks the Formación de Personal Investigador Programme (Ministerio de Ciencia e Innovación) for the financial support. This research was supported by the Spanish government under the Plan Nacional de I + D + I, co-financed by FEDER funds (project CGL-2009-08825), and the Junta de Andalucía, Consejería de Economía, Innovación y Ciencia (project CVI-7640). We also thank the Consejería de Medio Ambiente, Junta de Andalucía (Spain), for the permission to work in Sierra de Baza Natural Park. We sincerely thank Estefanía Berrio for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Varela-Cervero, S., López-García, Á., Barea, J.M. et al. Differences in the composition of arbuscular mycorrhizal fungal communities promoted by different propagule forms from a Mediterranean shrubland. Mycorrhiza 26, 489–496 (2016). https://doi.org/10.1007/s00572-016-0687-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-016-0687-2