Abstract
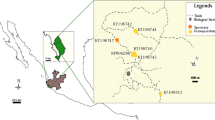
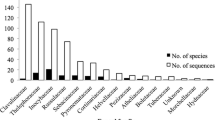
Ectomycorrhizal (ECM) fungi have a worldwide distribution. However, the ecology of tropical ECM fungi is poorly documented, limiting our understanding of the symbiotic associations between tropical plants and fungi. ECM Basidiomycete diversity was investigated for the first time in two tropical rain forests in Africa (Western Upper Guinea) and in Asia (Western Ghats, India), using a fragment of the mitochondrial large subunit rRNA gene to type 140 sporocarps and 54 ectomycorrhizas. To evaluate taxonomic diversity, phylogenetic analyses were performed, and 40 sequences included from identified European specimens were used as taxonomic benchmarks. Five clades were recovered corresponding to six taxonomic groups: boletoids, sclerodermatoids, russuloids, thelephoroids, and a clade grouping the Amanitaceae and Tricholomataceae families. Our results revealed that the Russulaceae species display a great diversity with several putative new species, especially in Guinea. Other taxonomic issues at family/section levels are also briefly discussed. This study provides preliminary insights into taxonomic diversity, ECM status, and biogeographic patterns of ECM fungi in tropical two rain forest ecosystems, which appear to be as diverse as in temperate and boreal forests.





Similar content being viewed by others
References
Alexander IJ, Högberg P (1986) Ectomycorrhizas of tropical angiospermous trees. New Phytol 102:541–549
Baura G, Szaro TM, Bruns TD (1992) Gastrosuillus laricinus is a recent derivative of Suillus grevilleii: molecular evidence. Mycologia 84:592–597
Berbee ML, Taylor JW (1993) Dating the evolutionary radiations of the true fungi. Can J Bot 71:1114–1127
Berbee ML, Taylor JW (2001) Fungal molecular evolution: gene trees and geologic time. In: McLaughlin DJ, McLaughlin RG, Lemke PA (eds) The Mycota VII. Part B. Systematics and evolution. Springer, Berlin, pp 229–245
Binder M, Hibbett DS (2002) Higher-level phylogenetic relationships of Homobasidiomycetes (Mushroom-forming fungi) inferred from four rDNA regions. Mol Phylogenet Evol 22:76–90
Bruns TD, Szaro TM (1992) Rate and mode differences between nuclear and mitochondrial small-subunit rRNA genes in mushrooms. Mol Biol Evol 9:836–855
Bruns TD, Szaro TM, Gardes M et al (1998) A sequence database for the identification of ectomycorrhizal Basidiomycetes by phylogenetic analysis. Mol Ecol 7:257–272
Bull JJ, Huelsenbeck PP, Cunningham CW, Swofford DL, Waddell PJ (1993) Partitioning and combining data in phylogenetic analysis. Syst Biol 42:384–397
Buyck B (1994a) Russula I (Russulaceae). Flore Illus Champignons Afr Cent 15:335–408
Buyck B (1994b) Russula II (Russulaceae). Flore Illus Champignons Afr Cent 16:411–542
Buyck B (1997) Russula III (Russulaceae). Flore Illus Champignons Afr Cent 17:545–597
Buyck B, Thoen D, Walting R (1996) Ectomycorrhizal fungi of the Guinea–Congo Region. Proc R Soc Edinb Sect B 104:313–333
Dahlberg A, Jonsson L, Nylund JE (1997) Species diversity and distribution of biomass above and belowground among ectomycorrhizal fungi in an old-growth Norway spruce forest in south Sweden. Can J Bot 75:1323–1335
Debaud JC, Marmeisse R, Gay G (1999) Intraspecific genetic variation and populations of ecomycorrhizal fungi. In: Varma AK, Hock B (eds) Mycorrhiza: structure, molecular biology and function. Springer, Berlin, pp 75–110
den Bakker HC, Zuccarello GC, Kuyper TH W, Noordeloos ME (2004) Evolution and host specificity in the ectomycorrhizal genus Leccinum. New Phytol 163:201–215
Doyle JJ (1992) Gene trees and species trees: molecular systematics as one-character taxonomy. Syst Bot 17:144–163
Eberhardt U (2002) Molecular analyses of the agaricoid Russulaceae: correspondence with mycorrhizal and sporocarp features in the genus Russula. Mycol Prog 1(2):201–224
Eberhardt U, Verbeken A (2004) Sequestrate Lactarius species from tropical Africa: L. angiocarpus sp. nov. and L. dolichocaulis comb. nov. Mycol Res 108:1042–1052
Egger KN (1995) Molecular analysis of ectomycorrhizal fungal communities. Can J Bot 73:S1415–S1422
Erland S, Jonsson T, Mahmood S, Finlay RD (1999) Below-ground ectomycorrhizal community structure in two Picea abies forests in southern Sweden. Scand J For Res 14:209–217
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for Basidiomycetes—application for the identification of mycorrhizas and rusts. Mol Ecol 2:113–118
Gardes M, Bruns TD (1996) Community structure of ectomycorrhizal fungi in a Pinus muricata forest: above and below-ground views. Can J Bot 74:1572–1583
Gardes M, White TJ, Fortin J, Bruns TD, Taylor JW (1991) Identification of indigenous and introduced symbiotic fungi in ectomycorrhizas by amplification of nuclear and mitochondrial ribosomal DNA. Can J Bot 69:180–190
Grogan P, Baar J, Bruns TD (2000) Below-ground ectomycorrhizal community structure in a recently burned bishop pine forest. J Ecol 88:1051–1062
Hibbett DS, Gilbert LB, Donoghue MJ (2000) Evolutionary instability of ectomycorrhizal symbioses in Basidiomycetes. Nature 407:506–508
Horton TR, Bruns TD (2001) The molecular revolution in ectomycorrhizal ecology: peeking into the black-box. Mol Ecol 10:1855–1871
Huelsenbeck JO, Rannala B (1997) Phylogenetic methods come of age: testing hypotheses in an evolutionary context. Science 276:227–232
Jonsson L, Dahlberg A, Nilsson M-C, Kårén O, Zackrisson O (1999) Continuity of ectomycorrhizal fungi in self-regenerating boreal Pinus sylvestris forests studied in comparing mycobiont diversity on seedlings and mature trees. New Phytol 142:151–162
Jonsson L, Dahlberg A, Brandrud T-E (2000) Spatiotemporal distribution of an ectomycorrhizal community in an oligotrophic Swedish Picea abies forest subjected to experimental nitrogen addition: above and below-ground views. For Ecol Manag 132:143–156
Kõljalg U, Dahlberg A, Taylor AFS et al (2000) Diversity and abundance of resupinate thelephoroid fungi as ectomycorrhizal symbionts in Swedish boreal forests. Mol Ecol 9:1985–1996
Kõljalg U, Larsson KH, Abarenkov K et al (2005) UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol 166:1063–1068
Kretzer AM, Dunham S, Molina R, Spatafora JW (2003) Microsatellite markers reveal the below ground distribution of genets in two species of Rhizopogon forming tuberculate ectomycorrhizas on Douglas fir. New Phytol 161:313–320
Lee SS, Alexander IJ, Watling R (1997) Ectomycorrhizas and putative ectomycorrhizal fungi of Shorea leprosula Miq. (Dipterocarpaceae). Mycorrhiza 7:63–81
Marx DH (1969) The influence of ectotrophic mycorrhizal fungi on the resistance of pine roots to pathogenic infections. I. Antagonism of mycorrhizal fungi to root pathogenic fungi and soil bacteria. Phytopathology 59:153–163
Maury-Lechon G, Curtet L (1998) Biogeography and evolutionary systematics of Dipterocarpaceae. In: Appanah S, TurnbullU MJ (eds) A review of dipterocarps taxonomy, ecology and silviculture. CIFOR, Jakarta, pp 5–44
Miller SL, Buyck B (2002) Molecular phylogeny of the genus Russula in Europe with a comparison of modern infrageneric classifications. Mycol Res 106:259–276
Molina R, Massicotte H, Trappe JM (1992) Specificity phenomena in mycorrhizal symbiosis: community—ecological consequences and practical implications. In: Allen MF (ed) Mycorrhizal functioning. Chapman & Hall, London, UK, pp 357–423
Moncalvo JM, Drehmel D, Vilgalys R (2000) Variation in modes and rates of evolution in nuclear and mitochondrial ribosomal DNA in the mushroom genus Amanita (Agaricales, Basidiomycota): phylogenetic implications. Mol Phylogenet Evol 16:48–63
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Natarajan K, Senthilarasu G, Kumaresan V, Rivière T (2005) Diversity in ectomycorrhizal fungi of a dipterocarp forest in Western Ghats. Curr Sci 88(12):1893–1895
Newbery DM, Alexander IJ, Rother LA (1997) Phosphorus dynamics in lowland African rain forest: the influence of ectomycorrhizal trees. Ecol Monogr 67:367–409
Nicholas KB, Nicholas HB, Deerfield DW II (1997) GeneDoc: analysis and visualization of genetic variation. Embnet News 4:1–4
Onguene NA, Kuyper TW (2001) Mycorrhizal associations in the rain forest of South Cameroon. For Ecol Manag 140:277–287
Pascal JP, Pélissier R (1996) Structure and floristic composition of a tropical evergreen forest in south-west India. J Trop Ecol 12:191–211
Pélissier R, Pascal JP, Houllier F, Laborde H (1998) Impact of selective logging on the dynamics of a low elevation dense moist evergreen forest in the Western Ghats (South India). For Ecol Manag 105:107–119
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Redhead JF (1980) Mycorrhiza in natural tropical forests. In: Mikola P (ed) Tropical mycorrhiza research. Clarendon, Oxford, pp 127–142
Rivière T (2004) Biodiversity, molecular ecology and phylogeography of tropical ectomycorrhizal symbiosis. Ph.D. thesis, Université de Montpellier II, France
Rivière T, Natarajan K, Dreyfus B (2005) Spatial distribution of ectomycorrhizal Basidiomycete Russula subsect. Foetentinae populations in a primary dipterocarp rainforest. Mycorrhiza 16:143–148
Romagnesi H (1985) Les Russules d’Europe et d’Afrique du Nord. J. Cramer, Lehre (reprint with supplement)
Sanon KB, Bâ AM, Dexheimer J (1997) Mycorrhizal status of some fungi fruiting beneath indigenous trees in Burkina Faso. For Ecol Manag 98:61–69
Shimono Y, Kato M, Takamatsu S (2004) Molecular phylogeny of Russulaceae (Basidiomycetes; Russulales) from the nucleotide sequences of nuclear large subunit rDNA. Mycoscience 45:303–316
Singer R (1986) The Agaricales in modern taxonomy, 4th edn. Koeltz Scientific, Koeningstein
Singer R, Araujo IJS (1979) Litter decomposition and ectomycorrhiza in Amazonian forests: 1. A comparison of litter decomposition and ectomycorrhizal Basidiomycetes in latosol–terra firme rain forest and white podzol Campinarana. Acta Amazon 9:25–41
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic, San Diego, CA
Smits WTM (1992) Mycorrhizal studies in dipterocarp forests in Indonesia. In: Read DJ, Lewis DH, Fitter AH, Alexander IJ (eds) Mycorrhizas in ecosystems. Cambridge, pp 283–292
Swofford DL (2001) PAUP‡: phylogenetic analysis using parsimony (‡and other methods), ver. 4. Sinauer, Sunderland, MA
Thoen D, Bâ AM (1989) Ectomycorrhizas and putative ectomycorrhizal fungi of Afzelia africana Sm. and Uapaca guineensis Müll. Arg. in southern Senegal. New Phytol 113:549–559
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Wall JD (2003) Estimating ancestral population sizes and divergence times. Genetics 163:395–404
Watling R, Lee SS (1995) Ectomycorrhizal fungi associated with members of the Dipterocarpaceae in Peninsular Malaysia. J Trop For Sci 7:657–669
White TJ, Bruns TD, Lee SS, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelf DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and application. Academic, San Diego, pp 315–322
Acknowledgements
We thank P. Deschères, G. Ifono (Eaux et Forêts, Guinée), A. Fontana (IRD, Guinée) for their help in identifying several tree species. We thank Dr. V. Kumaresan (CASB, India) for fruitful collaboration. The authors are grateful to Dr. D. McKey (CNRS, France) and Dr. O. Dangles (IRD, France) for useful comments, discussion, and support. This work was supported by a doctoral grant from the French Institute of Pondicherry to Taiana Rivière.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riviere, T., Diedhiou, A.G., Diabate, M. et al. Genetic diversity of ectomycorrhizal Basidiomycetes from African and Indian tropical rain forests. Mycorrhiza 17, 415–428 (2007). https://doi.org/10.1007/s00572-007-0117-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-007-0117-6