Abstract
A cost effective and reliable technology for the fabrication of electrochemical test-cell arrays for battery materials research, based on batch-fabricated glass micro packages was developed and tested. Jet dispensing was investigated for the first time as a process for fabricating battery electrode arrays and separators and compared to micro dispense printing. The process shows the reproducibility over the whole range of investigated materials and battery cell structures that is required for battery materials research. Such setup gives rise to a significantly improved reliability and reproducibility of electrochemical experiments. Cost-effective fabrication of our test chips by batch processing allows for their single-use in electrochemical experiments, thereby preventing contamination issues due to repeated use as in conventional laboratory test cells. In addition, the integration of micro pseudo reference electrodes is demonstrated. Thus, the test cell array together with the developed electrode/electrolyte deposition technology provide a highly efficient tool for speedy combinatorial and high throughput testing of battery materials on a system level (full cell tests). Experimental results are shown for the microfabrication of lithium-ion test cells with help of several electrode and binder materials. The influence of jetting parameters on electrode lateral dimensions and thickness, reproducibility of the electrode mass as well as the use of integrated micro-reference electrodes for impedance spectroscopy and cyclic voltammetry measurements in micro cells are presented in detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rechargeable batteries are of crucial importance for the transition towards a sustainable energy economy. Major applications of secondary batteries are for stationary storage of electricity from renewable sources, and batteries for electrical vehicles, leading to the new, demanding requirements. Here, both the discovery of new materials and the study and optimization of new and known materials can be accelerated significantly by high-throughput (HT) testing technologies (Muster et al. 2011).
Combinatorial and HT methods comprise rapid synthesis, high-throughput testing and high capacity information processing. In this context, “combinatorial” refers to a change in the nature of the parameters (different materials or components of a recipe) while HT means the systematic variation of parameters in a wide parameter space of a particular system with a given composition (Maier et al. 2007), both promising an acceleration of research. In addition, the case of testing several identical cells in parallel increases the statistical validation. However, in the known art, the implementation of combinatorial and HT methods in battery research may be difficult for several reasons. Huge variation of the electrochemical behavior of materials can occur even for very small variations of the material composition, in fact, impurities and additives of extremely low concentration can influence the electrochemical behavior greatly.
Moreover, the fabrication of battery electrodes are characterized by complex technologies like mixing which are not easily suited for the preparation of continuous material libraries. For example, synthesizing electrodes with continuous composition spreads were performed with alloy electrodes that were sputtered or evaporated simultaneously from two or more spatially separated sources (Alcock et al. 2011; Fleischauer et al. 2003; Jun et al. 2014; MacEachern et al. 2015; Whitacre et al. 2003). Unfortunately, this technology is not relevant for many interesting and industry relevant electrode materials.
HT methods were also used to screen polymer and liquid electrolytes for batteries (Cartier et al. 2015; Cekic-Laskovic et al. 2014; Su et al. 2014). Impressive results have been obtained recently with the help of commercial robotic liquid screening platforms with an automated mixing system (Cartier et al. 2015; Su et al. 2014), leading to detailed electrolyte libraries with different amounts of various organic solvents and various lithium salts and evaluating the ionic conductivity but not the performances of those electrolytes in battery cells was shown.
Although the research needs in the field of secondary batteries are high, and the large amount of possible electrode and electrolyte materials opens an almost infinite number of different cell configurations, actually a rather small number of publications report HT methods for the investigation of complete electrochemical cells, perhaps due to the complexity of the total setup, where test cell arrays with liquid electrolyte (Clemmons 2016; Cekic-Laskovic et al. 2014; Takada et al. 2004) are even more challenging to realize than cells with solid state thin film electrolyte (Alcock et al. 2011; Fleischauer et al. 2003; Whitacre et al. 2003). Thus, until now the great majority of battery experiments is conducted based on single cell preparation. Coin cells and pouch packaged cells are used for two-electrode measurements while three-electrode measurements are carried out with sophisticated mechanical constructions like T-cells (Garcia et al. 2016; Loveridge et al. 2016) or El-cells (Steinhauer et al. 2017).
This paper proposes a breakthrough in electrochemical testing methodology, by much wider use of arrays of battery cells for high throughput tests, which allow the parallelization of potentiometry, amperometry, and electrochemical impedance spectroscopy (EIS) experiments. This will speed up testing in order to evaluate electrochemical properties and correlate electrode/electrolyte variations for a large number of rationally modified cells. Our cells will be low cost, because hundreds of them can be fabricated on the same substrate with the help of Fraunhofer IZM´s glass panel fabrication line. Moreover, in contrast to the already existing in-house proprietary technologies of ILIKA (Alcock et al. 2011) and Wildcat Technologies (Takada et al. 2004; Zhu et al. 2016), we provide a complete platform for producing electrochemical test cell arrays, including HT devices intended for use as fully finished or as work-in-progress (partially finished) goods, to any interested lab.
Said technology is based on IZMs micro battery technology (Hahn et al. 2016, 2017; Hoeppner et al. 2015; Hoeppner 2015; Ferch et al. 2016) and comprises the following key components:
-
Fabrication of glass cavity substrates with area array of test cells, metallic thin-film or printed carbon current collectors, integrated micro-pseudo-reference electrodes (MPRE) and electrical contacts intended for single use,
-
Preparation of electrode pastes and fabrication of electrodes by conventional/industry relevant methods and sequential variation of material parameters,
-
Provision of electrode pastes as cartridges for dispense-print or jetting in commercial robot systems to be deposited into the cell array,
-
Housing of test-cell arrays or sub-arrays via a substrate bonding technology and assembly into re-usable test fixtures; electrolyte application can be variably done with the help of automated robotic systems or fabricated manually and supplied as cartridges, and
-
Provision of a reliable electrical interface to a multichannel electrochemical testing system for combinatorial characterization.
The main building blocks that are required for that approach are shown in Fig. 1a. They can be divided into two main parts: the high throughput battery cell array fabrication and the high throughput electrochemical characterization and data evaluation. We are using a multichannel battery test station (Basytec) but other concepts like multiplexed potentiostats and impedance analyzers can also be applied.
Building blocks of the high throughput battery cell test approach (a); filling of high vapor pressure electrolyte through a manifold that is integrated in the test array lid (b) and final array device for testing (c); battery cell test array for high throughput testing that uses variations of electrode material across the array columns and electrolyte variations across rows (d)
Due to the single–use nature of the test-cell arrays and the standardized assembling technology, failures arising from impurities and manual handling will be significantly reduced in comparison to reusable single cells (T-cells/Swagelok cells, or El-cells). Cells of the area-array-substrate concept can be made in various sizes and variants, allowing diverse measurements including 3-electrode electrochemical characterization, optical diagnosis and gas evolution. Despite their single-use nature, such test-cells are very cost-efficient: nearly 10,000 test cells of size 5 × 5 mm2 can be fabricated simultaneously on one glass substrate. The test cells can also be used for highly corrosive electrolytes, since the active materials are only in contact with the glass housing and the tested stable thin-film metallization. Materials can be identical across an array to document consistency from cell to cell, leading to higher precision and statistical significance; it can also help to evaluate the differences in electrochemical performance for a plurality of experiments ran in parallel with varying electrolyte, electrode composition or content.
Electrodes for conventional state-of-the-art test cells are fabricated similar to large scale manufacturing by slurry casting or doctor blading. A paste that is fabricated by mixing the powder components and binder into a solvent to make a slurry; the slurry mixture is pasted onto a metal foil; evaporating the solvent and then pressing (calendering) to finalize the electrode.
In order to deposit the patterned micro electrodes mooted here onto a substrate a process must be used that is suitable for the deposition of such high viscosity materials in small dimensions. Screen printing would be possible but paste additives are required to control the rheology; those additives can deteriorate the electrochemical performance. Thus dispensing is the most straight forward way to fabricate arrays of micro electrodes, where the viscosity required by the process can be achieved by variation of the solvent content and no other additives are needed (Ferch et al. 2016). As an alternative method we report here for the first time the use of high speed jetting technology for the deposition of battery electrode pastes. The jetting technology was developed for depositing high viscosity solder or silver pastes for the fabrication of solder bumps and interconnection lines (Becker et al. 2014; Gu et al. 2016): here, fluid is ejected rapidly through a nozzle, using said fluid´s momentum to break free from the nozzle. It is mostly used for small scale production; the main advantages over dispense printing is the much higher speed; moreover, there is no need for accurate control of the nozzle height because it is a contactless process.
The fundamentals of jet deposition are rather complex, in particular for battery electrode pastes, which are liquid–solid two-phase fluids that are composed of several components (ceramic and carbon particles, polymer binders and solvents). The many process parameters will not only influence deposition accuracy; but in many cases it is hard to find a parameter window that does not result in clogging of the nozzle orifice or even the damage of the electrode paste components under the high internal forces of the ejection system.
Therefore we conducted a design of experiments leading to a working jetting process for several anode, cathode and separator materials.
The dispensing and the jetting approach are suitable to evaluate materials in a form close to the battery production since the same electrode paste composition and particle sizes that are relevant for large scale production can be employed.
Both processes can support the combinatorial and high throughput approach because changing cartridges for different or modified material compositions is straight forward thus allowing to change material composition during the fabrication of a test cell array. This so called post-synthesis array transfer (PoSAT, Roberts et al. 2007) has the advantage of providing a robust composite structure, optimized for high electronic and ionic conductivities and a good contact with the current collector as in a real battery cell.
Reasonable test array confiurations are as follows:
-
deposition the same set of anode and cathode materials on the complete cell array. Those cells can be tested with programmed electrolyte variations provided by a conventional pipette robot system or they can be used with the same electrolyte but tested under different electrical conditions at the same time,
-
deposition of an electrode with varied composition in each row (column) of the array and a variation of electrolyte in each column (row) of the array,
-
anode versus cathode variations over the rows/columns of the array is possible in the same way.
Pipetting of liquid electrolyte into such test cell arrays is straight forward only in case of low vapor pressure fluids. Unfortunately conventional electrolytes of state of the art lithium-ion batteries use high vapor pressure organic solvents. For this type of tests we designed a particular set-up that uses the cell sealing plate for electrolyte filling as shown in Fig. 1b, c. For that purpose a fluidic manifold is integrated into the sealing lid of the test cell array that allow to supply the same electrolyte into each row of the array while the electrodes are varied over the columns. The manifold channel is dried after electrolyte supply to avoid ionic short circuit between the individual test cells.
Another advantage is the possibility to integrate MPREs during the micro-fabrication process or the dispense-print process with nearly no extra cost. Several types of pseudo-reference electrodes have been proven to exhibit long-term reference-potential stability in relevant electrochemical systems. They are essential for performing precise and reproducible electrochemical measurements (Bonnaud et al. 2016; Ives 1961; La Mantia et al. 2013; Ruch et al. 2009; Weingarth et al. 2012a, b; Zhou and Notten 2004). Lithium (Zhou and Notten 2004) and partially lithiated lithium titanate (LTO) (La Mantia et al. 2013) can be used as reference electrodes for lithium-ion batteries with organic electrolytes. Metallic lithium can be deposited by electroplating with room-temperature ionic liquid (RTIL) electrolyte. Porous carbonaceous materials and platinum have also been tested as reference electrodes in ionic liquid electrolytes (Bonnaud et al. 2016). Porous carbon electrodes can be fabricated by electrophoretic deposition, dispensing or spray deposition. Those carbonaceous materials had a low potential drift over days as well as a high tolerance for impurities (Maminska et al. 2006; Saheb et al. 2006; Weingarth et al. 2012a, b; Widmaier et al. 2016). We here investigated the use of LTO MPREs for lithium-ion cells; since LTO is one of the anode materials, it requires no additional fabrication effort.
2 Experimental
2.1 Fabrication of substrates for the battery test cell arrays
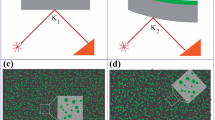
Cell designs for co-planar (interdigitated) and stacked (face-to-face) electrodes with and without reference electrodes, as well as other technological variants are shown in Fig. 2. A third substrate (encapsulation lid, not shown in Fig. 2) is usually bonded to the top substrate to close the electrolyte fill holes. Cells of the side-by-side electrode design (Fig. 2c, d), where all active materials are located in the bottom substrate, are truly much easier to fabricate, while cells with the conventional stacked design—as in all commercial batteries (Fig. 2a, b)—require printing of a separator and a careful thickness adjustment of the deposited active materials.
Substrate integrated test-cell configurations: stacked or face-to-face electrodes (a, b), side-by-side or interdigitated electrodes (c, d). Where 1: top substrate with cavities and electrolyte filling holes (8), 2: metal foil substrate, 3: bottom substrate with cavities and metallization, 4: polymer cover with cavity and electrolyte fill holes, 5: anode (or cathode), 6: cathode (or anode), 7: separator, 8: hole, 9,10: current collectors, 11: electrolyte, 12: reference electrode, 13: adhesive bond, 14: electrically conducting adhesive, 15: elastomer gasket, 20: electrical contacts
The cavities and holes in the glass substrates were fabricated by laser-ablation or alternatively by wet chemical etching and sandblasting. A MDI LD600-H panel level laser system in combination with a green short-pulse laser (edgewave BX-series, wavelength of 532 nm, pulse lengths from 5 ns) with a maximum power of 20 watts was used for substrate patterning at Fraunhofer IZM. This system offers the possibility to directly structure glass substrates from 0.1 to 10 mm thickness up to a panel size of 610 × 450 mm2 with a high flexibility regarding the contour (layout-based). No mask or other equipment was necessary. The layer-by-layer removal sequence permits 2.5D machining with a lateral and orthogonal accuracy of < 20 μm and < 100 μm, respectively. The smallest possible structure width is approximately 100 μm. Etched glass cavities of 200 µm depth as well as laser patterned and metallized glass substrates are shown in Fig. 3a, b, respectively. The metallization of current collectors (Ti, Pt, Au, Cu, Ni, Al) is done by sputtering and lithography. Indium, tin, nickel and zinc can be electroplated using the semi-additive technology. Proven thin-film metallization systems as current collectors are available for lithium-ion and aluminum-ion as well as for aqueous Zn–MnO2 batteries. For cell configurations according to Fig. 2a, electrodes can be directly deposited on metal foils.
2.2 Electrode fabrication
The electrode materials were deposited into the cavities with the precision dispenser and jetting heads of Musashi Shotmaster 300 robot platform. Super Sigma CMII VS and Aerojet MJET-A devices have been used for the dispensing and jetting, respectively (Fig. 4). With such devices, a wide variety of electrode pastes of different characteristics can be deposited. This allows to test several electrode pastes to select the best for optimum battery production. High speed deposition is possible with the Aerojet print head that is capable of 330 shots per second even for highly-viscous pastes.
Calendering (mechanical compressing) of electrodes cannot be done in cavity substrates. However it is an important process in industrial battery manufacturing controlling the electrode thickness and pore size. If calendering of electrodes has to be investigated, the configuration shown in Fig. 2a can be applied where the electrode of one side is deposited on a planar metal foil. Here, dispensing, and jetting can be used to prepare the electrodes on the metal foil. After mechanical pressing the electrode foil may be cut to the size of the array substrate and fixed on a carrier substrate. In that case, all bottom electrodes are electrically interconnected. A carbon coating can be optionally deposited prior to electrode fabrication on metal foils or thin film metallization by dispensing and jetting.
Polyvinylidene fluride (PVDF) is currently the most used binder for lithium-ion battery electrode fabrication. For environmental and cost reasons, water based binders are also under investigation (Wood et al. 2017). Because aqueous systems influence not only the electrode chemistry—in particular on the positive electrode side—but also paste rheology and mixing characteristics, water based binders were also tested for the deposition of micro-structured electrodes. All the electrode and separator materials used here and the obtained electrode properties are summarized in Table 1. The ratio in weight percent of active material, binder and carbon black (C65 from Imerys) is 90:7:3, 90:5:5 and 92:4:4 for graphite, LTO and the cathode, respectively. Following the addition of the solvent (n-methylpyrrolodone (NMP) for the PVDF binder and water for the carboxymethl cellulose/styrene-butadiene rubber (CMC/SBR) binder), the mixture was iteratively subjected to speed mixing and ultra-sonication for several times until a homogeneous electrode slurry suitable for micro-dispensing was achieved. Low agglomerate size of particles is crucial for dispensing or jetting; the resulting maximum agglomerate sizes were in the range of 10–25 µm (Table 1). This is an important parameter that influences processing and electrode performance. The minimum electrode thickness should be ca. 50 µm (two times the particle size) and the minimum inner diameter of the dispensing needle or jet nozzle should be ca. 250 µm (ten times the particle size).
It has been shown previously, that cells with the side-by-side design are characterized by the same current capability and comparable electrochemical characteristic in respect to the conventional face-to-face configuration, if the distance between anode and cathode is small (30–100 µm) and the top electrolyte layer is sufficiently thick (Hoeppner 2015).
Such cells based on glass housing also enable a range of new analytic tools for studying batteries by optical methods.
2.3 Assembly and electrolyte filling
Room-temperature adhesive bonding applying a special UV-adhesive with a high barrier function against water vapor is applied to bond top and bottom substrates and seal the cell arrays. This adhesive is stable for the electrolytes used for lithium-ion batteries. For other more aggressive electrolytes, a per-fluorinated elastomer gasket and a polymer top plate (polyether ether ketone—PEEK) are used (Fig. 2d). Printing of a separator on top of the electrode is essential for the stacked battery configurations (Fig. 2a, b). Separator pastes and a technology for dispense-printing of patterned separators were developed based on SiO2 particles and appropriate binders (Ferch et al. 2016). Electrolyte filling can be done with a dispense robot in case of RTIL electrolytes because they are characterized by nearly zero vapor pressure. Dispensing of the organic carbonate electrolytes is not possible or requires a pressure chamber and cooled substrates because the organic solvent electrolyte formulations evaporate easily at room temperature. Instead of using a pressurized containment, we deployed a top sealing plate of the test fixtures with an integrated electrolyte fill channel as shown in Fig. 1. Gaskets are integrated into the sealing plate. For each row (stripe) of the test-cell array, the electrolyte channel is connected to a vacuum pump and electrolyte syringe. Thus, the test cells can be evacuated prior to electrolyte filling.
2.4 Cell design
The test cell substrate of the prototype system is divided into single chips of 10 × 15 mm2 at a pitch of 10.3 and 15.3 mm in the x- and y- direction, respectively (Fig. 2a). Each chip contains one, two or four electrochemical test cells, four holes for electrolyte filling and 10 electrical contacts. The active cell area of the stacked electrodes is 6 × 8 mm2 or 3.6 × 4.8 mm2; the size of one electrode stripe for the interdigitated cells is 8 × 0.6 mm2. Final sealing and electrolyte filling can be made with single chips or stripes of 8 chips of size 82.1 × 15 mm2 (Fig. 2b). That configuration enables straightforward manual handling and mechanical adjustment.
2.5 Electrical Characterization
The test-cell arrays with several micro-electrodes require a reliable electrical interconnection system. Figure 3c shows electrical test fixtures for array stripe substrates with 80 electrical contacts each and electrolyte fill openings. Gold-coated springs were used to contact the current collectors on the test-chip substrates. Electrical characterization of the test-cell arrays was done employing a Basytec CTS-XL and Maccor multichannel battery tester with a total capacity of 400 channels. Electrochemical impedance spectroscopy and cyclic voltammetry were carried out with Bio-Logic SP-300 potentiostat/galvanostat equipped with a frequency response analyzer. The frequency range was 500 kHz–200 mHz with 10 mV AC voltage amplitude.
3 Results
3.1 Electrode fabrication for lithium-ion micro cell arrays
The viscosity of the electrode pastes were measured as a function of the NMP content. Figure 5a shows the shear rate dependent viscosities at 25 °C of LTO pastes with a solid content between 38 and 48%. It is quite similar to typical solder pastes (Becker et al. 2014). The viscosity can be reduced by adding more solvent to the paste. All pastes show a similar shear thinning behavior over the whole range of shear rates from 10−1 to 10 s−1. Shear thinning at higher shear rates is typical for dispensing or jetting through narrow nozzles. With the help of the described paste mixing procedure and the solvent content adjustments, it was possible to achieve the dispensing or jetting of the chosen electrode materials.
While good results can be easily obtained, in the case of dispensing, by only adjusting the dispense pressure and the distance between needle and substrate, much more parameters had to be investigated for the jetting process. The most important parameter is the nozzle orifice diameter but the shot time, tappet stroke, chamber supply pressure, distance to the substrate and temperature also influence the jetting process significantly. The time indicated in Fig. 5b, c defines the duration of the tapped raised. The height and diameter of the deposited droplets (depots) were measured for ca. 20 drops of each material and parameter configuration. The influence of tappet stroke, nozzle diameter and stroke time for the graphite paste is shown in Fig. 5b, c. The tappet stroke has only a minor influence on the droplet volume but it significantly affects the speed of ejection. A higher stroke is required for pastes with higher viscosity. Too high stroke can lead to splashing of the material on the substrate and formation of satellite deposits. The amount of deposited material is not dependent on the distance between substrate and nozzle but higher distances reduce the lateral deposition accuracy. The minimum nozzle orifice diameter that allowed deposition without clogging was 220 µm and resulted in a depot thickness between 60 and 90 µm. This defines the minimum electrode thickness that can be fabricated by jetting. Slightly thinner layers can be deposited with conventional dispensing of the same material. A large, and strongly non-linear influence of depot size was investigated for the time, in particular for the 400 µm nozzle orifice diameter. A ten percent increase of the minimum time of 1 ms to 1.1 ms has much more effect than further doubling the time to 2 ms. This effect can be understood by the construction of the jetting system. It includes a chamber with nozzle of defined volume that is filled with the paste from the cartridge prior to the jetting event. At the smallest time the chamber/nozzle is not completely filled with material but at ca. 2 ms the chamber is nearly entirely filled and further increase of time will not further increase the amount of paste and therefore the drop volume.
As can be seen from Fig. 5b the smallest jetted dot diameter in case of graphite is 750 µm. Although, in practice we have seen that filling of 0.6 mm channels of the interdigitated battery cell design is feasible for some materials. The reproducibility of the jetted deposits is another important aspect. As can be seen in Fig. 6, graphite (g) and separator (i) deposits show a much better consistency than NCA (h). We were unable to find a paste material and jetting parameter combination for sufficient NCA electrode jetting quality. Figure 6e and f demonstrate how a continuous electrode can be jetted by reducing the distance between the deposits; that can be easily achieved by adjusting dispensing frequency and robot feed speed. Although single jetted deposits of the separator were quite homogeneous, the deposition of the separator on top of the electrode resulted in larger height fluctuation (Fig. 6k). More homogeneous separator layers were fabricated with help of the dispensing process (Fig. 6d). The dispensing of LTO and NMC electrodes on metal current collectors with geometrical features is demonstrated in Fig. 6b, d, respectively. We programmed an algorithm for the dispensing system that calculates the meandering horizontal traverse way for any geometrical dimensions (Fig. 6a).
Photographs of electrodes printed on metal foils with help of dispensing (a–d) and jetting (e–k); dispensing track for irregular electrode dimensions (a); LTO (b), NMC (c) and separator on NMC (d) dispense printing; jetting of carbon with uncomplete (e) and complete (f) surface coverage; single jet shots of graphite (g), NCA (h) and separator (i); full coverage of separator jetted on NCA electrode (k)
Prior to start of test cell printing experiments we validated the influence of the dispense process on the electrode performance. Electrodes were deposited by both, doctor blading and dispensing of meandering tracks on current collector foils and characterized in half cell tests with help of El-Cell test cells with polymer separator. As can be seen in Fig. 7e the four dispense printed cells are characterized by high reproducibility and show the same capacity like the doctor-bladed sample at low current (low C-rate). At high current (C-rates > 3C) the obtained capacity is slightly lower compared to the conventional cell fabrication but the performance is fully sufficient to use the printing technology for the high throughput test cell array concept. Figure 7a–c show some statistics of the test runs made with all the tested battery materials. For measuring the deposited electrode weight of the micro cells; here 10 … 16 cells were fabricated in single cell housings because individual weight measurement is not possible on the array substrate. As can be seen in Fig. 7b we were able to reduce the standard deviation of electrode mass from 10 … 17% (left values in each column) to 2 … 9% for the last deposition runs (right values in each column) due to fine tuning of parameters. Pastes with PVDF binder are characterized by slightly lower scattering compared to material with CMC binder. No difference was observed between the interdigitated electrode design (according to Fig. 2c) and stacked configuration (according to Fig. 2a). For proper balancing of the electrodes in the full cell an electrode mass variations between 2 and 9% seems to be rather high, but it can be compensated by a higher number of identical cells of each parameter combination fabricated in the array. An electrode thickness between 40 and 120 µm can be achieved with single deposition while 130 … 220 thick micro electrodes were fabricated with help of two deposition steps. Thus, area capacities between ca. 1 and ca. 5 mAh/cm2 can be obtained for the test chip arrays. The capacity distribution of the individual test planar graphite-NCA test cells (3.6 × 4.8 mm2) as well as interdigitated graphite-NCA and LTO-NCA cells (nine stripes 8 × 0.6 mm2) are shown in Fig. 7d. The focus of this work was to find parameters for stable printing of the thinnest possible electrodes for smallest possible test cells; thicker electrodes can be fabricated for larger test cells easily.
Electrode print statistics of several fabrication runs each including 10–16 cells: mean electrode mass (a), standard deviation of electrode mass of the two binder systems (b) and electrode thickness (c); discharge capacities of two test-cell fabrication runs with either the interdigitated (labelled as PVDF-c) or planar (labelled as PVDF-a) electrode design (d); specific discharge capacity as a function of current (C-rate) obtained from half-cell measurements on the fabricated NMC electrodes (e), with a comparison of a doctor bladed electrode and the dispensed (print 1–4) electrodes
3.2 Test of micro reference electrodes
One important feature of the micro-technology used for the test-cell fabrication is the straight forward integration of pseudo-reference electrodes for advanced electrochemical characterization; this has been demonstrated here by making impedance spectroscopy and cyclic voltammetry (CV) in the 3-electrode configuration. The basic principle of a CV experiment is shown in Fig. 8a, b. In a CV experiment, the working electrode (WE) potential is repeatedly shifted between two vertex potentials (V1 and V2) at a fixed potential sweep using a potentiostat. The reference point for controlling the working electrode potential is provided by the introduction of a non-polarizable reference electrode (RE) that does not take part in the electrochemical reaction and provides a stable reference potential. The current flows between the working (WE) and the counter electrode (CE). With CV experiments, one can obtain valuable information on the kinetics of electrochemical reactions as well as measure the contributions of anode and cathode to the overall cell potential at any time during the experiment. We have proven the feasibility of such experiments in a micro-test-cell by using a micro-glass battery cell with aluminum current collectors, and LTO and NCA as the anode and the cathode material, respectively. The reference potential was provided by an integrated pseudo-micro-reference electrode (MPREs) made of LTO (labelled 3 in Fig. 8c) that was arranged parallel with anode and cathode stripes (labelled 2 and 1 in Fig. 8c) and conditioned to a stable reduction potential by insertion of Li-ions prior to the experiment. We started the experiments with much smaller MPREs (0.6 × 0.6 mm2) but in this case the reference potentials were not stable; thus an MPRE of 0.6 × 8 mm2 sized is a good compromise between accuracy and space requirement in the micro-cell.
Since the stability of the potential of the reference electrode is crucial for such experiments, prior to the CV, the stability of the LTO reference electrode was measured, and the fluctuations were indeed smaller than 1 mV/h. This is sufficiently stable even for experiments with small sweep rates (≤ 1 mV/s). The CV was carried out on the micro-battery at normal laboratory atmosphere and room temperature with vertex potentials of 1.8 and 3 V, respectively, and a potential sweep of 0.1 mV per second using the biologic SP 300 potentiostat. Thus, the duration of one CV cycle is 6.7 h. After 5 CV cycles, a stable current response was obtained. Figure 8d–f shows the full-cell as well as the half-cell (anodic and cathodic) voltammograms obtained for the micro-battery during the 6th cycle. The CV plots show that the cathode predominates the overall cell potential in this specific case.
The results of the impedance spectroscopy of two test-cells according to the schematic in Fig. 8c are shown in Fig. 9. The frequency range was 500 kHz–200 mHz with 10 mV AC voltage amplitude. In both cases, the impedance contribution of the anode is higher than that of the cathode. This may be due to much larger active surface area of the LTO anode particles compared to the cathode material. Thus, the feasibility of standard electrochemical characterization of full cells with integrated reference electrode was demonstrated on the cell array level.
Impedance spectroscopy of two micro-cells with LTO and NCA electrodes using LTO stripe as MPRE according to the schematic of Fig. 8c
4 Conclusions and outlook
A high-throughput testing platform has been developed based on Fraunhofer IZM’s glass panel micro-patterning technology. Glass-based micro-test-cells and test-cell arrays have been batch-fabricated and assembled to achieve full electrochemical test-cells successfully. Fabrication of electrode pastes and electrodes by conventional/industry relevant methods and sequential variation of material parameters is possible. Micro dispensing and jetting were investigated as means for the standardized application of different electrode materials and SiO2-based separators. The reproducibility of the process was increased during the project significantly for the whole range of investigated materials and can be adapted for any material; PVDF and CMC-SBR binders were used. Jetting is a high speed deposition alternative for feature sizes above ca. 0.6–1 mm but showed reproducible results only for some electrode materials (graphite, LTO). Electrode mass variation of the micro-cells was 5–10% which is sufficient for many basic investigations and practical battery material optimizations, but still have to be improved for the optimization of production-relevant battery systems. By deposition of micro-structured metals and LTO micro-electrodes in electrochemical test-cells, micro-pseudo-reference electrodes of high stability were applied with high geometric and structural accuracy as provided by the micro-system technology processing. The application of CV and EIS with such micro-reference electrodes was demonstrated. Such test setup gives rise to an improved reliability and reproducibility of electrochemical experiments. The economic fabrication of our test arrays by batch processing allows their single-use in electrochemical experiments, preventing contamination issues due to repeated use as in conventional laboratory test cells. Clean room processing prevents particulate contamination and high grade purified material can be used cost-efficient for all experiments since only very small material volumes are required. For implementation of the substrate integrated cell arrays, a reusable electrical interface to multichannel electrochemical testing system was developed. Thus, the presented test-cell arrays can be efficiently deployed for screening tests of battery materials at the level of full-cells and are expected to speed up electrochemical materials research significantly. As a next step, standardized arrays and array interfaces have to be developed that are optimized for specific materials research tasks. They can include only the array substrates, substrates with electrodes or full-cells including electrolyte that will be made available. In addition, the glass substrate option allows these cells to be optically accessible which can be exploited for various in situ measurement methods.
References
Alcock HJ, White OC, Jegelevicius G, Roberts MR, Owen JR (2011) New high-throughput methods of investigating polymer electrolytes. J Power Sources 196:3355–3359. https://doi.org/10.1016/j.jpowsour.2010.11.098
Becker KF, Koch M, Voges, S et al (2014) Precision jetting of solder paste—a vesatile tool for small volume producion. In: International symposium on microelectronics, vol 1, pp 000438–000443
Bonnaud C, Billard I, Papaiconomou N, Chainet E, Lepretre JC (2016) Rationale for the implementation of reference electrodes in ionic liquids. Phys Chem Chem Phys 18:8148–8157. https://doi.org/10.1039/C5CP07652H
Cartier C, Feng Z, Faulk J, Scherson D (2015) A combinatorial approach toward the discovery of electrolyte formulations for non-aqueous electrochemical energy storage devices. ECS Electrochem Lett 4:A110–A114. https://doi.org/10.1149/2.0081509eel
Cekic-Laskovic SN, Röser S, Winter M (2014) High throughput screening: unique feature of the electrolyte laboratory for fast, automated preparation and analysis of interesting combinations of materials in lithium and lithium-ion batteries. Kraftwerk Batterie Fachtagung, Münster
Clemmons O (2016) High throughput in-situ analysis of gas evolution in lithium ion batteries. In: 229th ECS Meeting San Diego, CA, 2016
Ferch M, Molnar M, Marquardt K, Hoeppner K, Luecking M, Elia G A, Buk J, Hahn R (2016) Segmented rechargeable micro battery for wearable applications based on printed separator and LTO/NMC electrodes. In: E-MRS spring meeting, Lille 2016
Fleischauer MD et al (2003) Design and testing of a 64-channel combinatorial electrochemical cell. J Electrochem Soc 150:A1465–A1469. https://doi.org/10.1149/1.1613670
Garcia G, Schuhmann W, Ventosa E (2016) A three-electrode battery-type Swagelok cell for the evaluation of secondary alkaline batteries: the case of the Ni–Zn battery. Chemelectrochem 3:592–597. https://doi.org/10.1002/celc.201500474
Gu S, Jiao X, Liu J, Yang Z et al (2016) Design and experiment of a solder paste jetting system driven by a piezoelectric stack. Micromachines 7:112–124. https://doi.org/10.3390/mi7070112
Hahn R, Molnar M, Ferch M, Lücking M, Hubl M, Elia G, Marquardt K (2016) Micro battery prototype line and micro fluidic electrolyte handling. In: IDTechEx energy harvesting and storage Europe Berlin
Hahn R, Ferch M, Hoeppner K, Queisser M, Marquardt K, Elia GA (2017) Development of micro batteries based on micro fluidic MEMS packaging. In: 2017 Symposium on design, test, integration and packaging of MEMS/MOEMS (DTIP), Bordeaux May 29 2017–June 1 2017, pp 1–5. https://doi.org/10.1109/dtip.2017.7984497
Hoeppner K et al (2015) Design, fabrication, and testing of silicon-integrated li-ion secondary micro batteries with interdigital electrodes. In: 15th International conference on micro and nanotechnology for power generation and energy conversion applications, vol 660,. Journal of Physics Conference Series
Hoeppner K, Marquardt K, Hahn R, Mukhopadhyay (2015) B Silicon-integrated secondary Li-ion micro batteries with side-by-side electrodes for the application as buffers in self-sufficient energy harvesting micro systems. In: 225th ECS meeting, Orlando, US, 2015.https://doi.org/10.1088/1742-6596/660/1/012064
Ives DJG (1961) Reference electrodes: theory and practice. Academic Press, Cambridge
Jun YJ, Park SH, Woo SI (2014) Combinatorial high-throughput optical screening of high performance Pd alloy cathode for hybrid Li-air battery Acs combinatorial. Science 16:670–677. https://doi.org/10.1021/co500041n
La Mantia F, Wessells CD, Deshazer HD, Cui Y (2013) Reliable reference electrodes for lithium-ion batteries. Electrochem Commun 31:141–144. https://doi.org/10.1016/j.elecom.2013.03.015
Loveridge MJ et al (2016) Towards high capacity Li-ion batteries based on silicon-graphene composite anodes and sub-micron V-doped LiFePO4 Cathodes. Sci Rep. https://doi.org/10.1038/srep37787
MacEachern L, Dunlap RA, Obrovac MN (2015) A combinatorial investigation of Fe–Si–Zn thin film negative electrodes for Li-ion batteries. J Electrochem Soc 162:A229–A234. https://doi.org/10.1149/2.1051501jes
Maier WF, Stoewe K, Sieg S (2007) Combinatorial and high-throughput materials science. Angew Chem Int Ed 46:6016–6067. https://doi.org/10.1002/anie.200603675
Maminska R, Dybko A, Wroblewski W (2006) All-solid-state miniaturised planar reference electrodes based on ionic liquids. Sens Actuators B Chem 115:552–557. https://doi.org/10.1016/j.snb.2005.10.018
Muster TH et al (2011) A review of high throughput and combinatorial electrochemistry. Electrochim Acta 56:9679–9699. https://doi.org/10.1016/j.electacta.2011.09.003
Roberts MR, Spong AD, Vitins G, Owen JR (2007) High throughput screening of the effect of carbon coating in LiFePO4 electrodes. J Electrochem Soc 154(10):A921–A928
Ruch PW, Cericola D, Hahn M, Koetz R, Wokaun A (2009) On the use of activated carbon as a quasi-reference electrode in non-aqueous electrolyte solutions. J Electroanal Chem 636:128–131. https://doi.org/10.1016/j.jelechem.2009.09.007
Saheb A, Janata J, Josowicz M (2006) Reference electrode for ionic liquids. Electroanalysis 18:405–409. https://doi.org/10.1002/elan.200503435
Steinhauer M, Diemant T, Heim C, Behm RJ, Wagner N, Friedrich KA (2017) Insights into solid electrolyte interphase formation on alternative anode materials in lithium-ion batteries. J Appl Electrochem 47:249–259. https://doi.org/10.1007/s10800-016-1032-3
Su L, Ferrandon M, Kowalski JA, Vaughey JT, Brushett FR (2014) Electrolyte development for non-aqueous redox flow batteries using a high-throughput screening platform. J Electrochem Soc 161:A1905–A1914. https://doi.org/10.1149/2.0811412jes
Takada K, Fujimoto K, Sasaki T, Watanabe M (2004) Combinatorial electrode array for high-throughput evaluation of combinatorial library for electrode materials. Appl Surf Sci 223:210–213. https://doi.org/10.1016/s0169-4332(03)00924-3
Weingarth D, Foelske-Schmitz A, Wokaun A, Koetz R (2012a) PTFE bound activated carbon-A quasi-reference electrode for ionic liquids. Electrochem Commun 18:116–118. https://doi.org/10.1016/j.elecom.2012.02.040
Weingarth D, Foelske-Schmitz A, Wokaun A, Kötz R (2012b) PTFE bound activated carbon—a quasi reference electrode for ionic liquids and its application. ECS Trans 50(11):111–117. https://doi.org/10.1149/05011.0111ecst
Whitacre JF, West WC, Ratnakumar BV (2003) A combinatorial study of LiyMnxNi2–xO4 cathode materials using microfabricated solid-state electrochemical cells. J Electrochem Soc 150:A1676–A1683. https://doi.org/10.1149/1.1622957
Widmaier M, Krüner B, Jäckel N, Aslan M, Fleischmann S, Engel C, Presser V (2016) Carbon as quasi-reference electrode in unconventional lithium-salt containing electrolytes for hybrid battery/supercapacitor devices. J Electrochem Soc 163:A2956–A2964. https://doi.org/10.1149/2.0421614jes
Wood D, Quass J, Li J, Ahmed S, Ventola D, Daniel C (2017) Technical and economic analysis of solvent-based lithium-ion electrode drying with water and NMP. Drying Technol 11(2017):1–11. https://doi.org/10.1080/07373937.2017.1319855
Zhou J, Notten PHL (2004) Development of reliable lithium microreference electrodes for long-term in situ studies of lithium-based battery systems. J Electrochem Soc 151:A2173–A2179. https://doi.org/10.1149/1.1813652
Zhu Y, Yang J, Cheng G, Carroll K, Clemons O, Strand D (2016) Novel non-carbonate based electrolytes for silicon anodes. Technical report WTD-DOE-1 2016-09-09. https://doi.org/10.2172/1351982
Acknowledgements
This work is part of the MATFLEXEND project funded by the European Union under contract FP7 604093 (www.matflexend.eu), the H2020 ALION project (www.alionproject.eu) under contract 646286 and the German Federal Ministry of Education and Research in the AlSiBat project under contract 03SF0486.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hahn, R., Ferch, M., Tribowski, K. et al. High-throughput battery materials testing based on test cell arrays and dispense/jet printed electrodes. Microsyst Technol 25, 1137–1149 (2019). https://doi.org/10.1007/s00542-019-04368-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-019-04368-5