Abstract
At present, there is no objective and absolute measure of nociception, although various monitoring techniques have been developed. One such technique is the Analgesia Nociception Index (ANI), which is calculated from heart rate variability that reflects the relative parasympathetic tone. ANI is expressed on a non-unit scale of 0–100 (100 indicates maximal relative parasympathetic tone). Several studies indicated that ANI-guided anesthesia may help reduce intraoperative opioid use. The usefulness of ANI in the intensive care unit (ICU) and during surgery has also been reported. However, some limitations of ANI have also been reported; for example, ANI is affected by emotions and some drugs. In 2022, a high frequency variability index (HFVI), which was renamed from ANI and uses the same algorithm as ANI, was commercialized; therefore, ANI/HFVI are currently in the spotlight. Unlike ANI, HFVI can be displayed along with other biometric information on the Root® monitor. ANI/HFVI monitoring may affect the prognosis of not only patients in the perioperative period but those in ICU, those who receive home medical care, or outpatients. In this article, we present an updated review on ANI that has been published in the last decade, introduce HFVI, and discuss the outlooks of ANI/HFVI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain is one of the utmost concerns for patients. A person’s report of an experience as pain should be respected [1]. However, there to date is no objective and absolute measure of nociception and pain [2], and there is no gold standard to quantify nociception [3]. To evaluate pain intensity, several subjective and numerical indications [e.g., Numerical Rating Scale (NRS), Visual Analogue Scale (VAS)] are commonly used in the clinical setting. In these indications, patients evaluate pain themselves; for example, in NRS, they score their pain from 0 (no pain) to 10 (worst pain imaginable). However, the NRS/VAS cannot be used during general anesthesia or when consciousness is impaired (e.g., during sedation, in severely ill patients, and in pediatric patients). Of course, inability to communicate does not negate the possibility that a human experiences pain [1]. In these patients, fluctuations of blood pressure and heart rate in response to nociceptive stimuli, several scores evaluated by medical staff, such as the Behavioral Pain Scale (BPS) and the Face, Legs, Activity, Cry, and Consolability (FLACC) scale [4], in combination with the staff’s own experience and intuition, are used to estimate nociception/pain. However, because these indicators include some subjectivity on the part of the evaluator, they cannot be said to be a complete objective evaluation of nociception/pain.
To address this issue, various nociception monitoring techniques have been developed [2, 5], such as Skin Conductance (MedStorm innovations, AS, Oslo, Norway) [6], Pupillometry (IDMED, Marseille, France) [7], Surgical Pleth Index (SPI, GE Healthcare, Helsinki, Finland) [8], nociception index (NOL, Medasense, Ramat Gan, Israel) [9], and Analgesia Nociception Index (ANI, Mdoloris Medical Systems, Loos, France). These utilize changes in the activation of sympathetic activity or decreases in relative parasympathetic tone that occur as a response to noxious stimuli [2]. Although many studies have been conducted to investigate these nociception monitoring techniques, a standard nociception/pain index has not been established to date, and none are widespread in many facilities.
Very recently, the high frequency variability index (HFVI, Mdoloris Medical Systems), which can be used to monitor relative parasympathetic tone, has appeared on the market in Japan. While ANI is exclusively for pain monitoring, the HFVI may be applied to other evaluation targets although they use the same algorithm. Therefore, understanding ANI is essential to achieve mastery of the HFVI. Since many studies on ANI have been conducted in various fields, and there are some previous reviews on ANI, in the present article, we present an updated review on articles on ANI that have been published in the last decade, introduce the HFVI, and discuss the outlooks of both ANI and HFVI.
The studies addressed in this narrative review were searched using a common electronic database (PubMed) about the ANI and HFVI published in 2011–2022. The keywords “analgesia nociception index” and “high frequency variability index” were used to find 226 potential articles from the database. One of the authors (KY) assessed the title, abstract, and full text of the articles. Since it is not possible to review all studies published in the last decade, randomized controlled trials and clinical studies with large numbers of subjects were prioritized and included in this review as key papers. We also included some papers that we judged to have important implications.
Although the two terms ‘nociception’ and ‘pain’ are sometimes confused, they have different meanings. The former is a physiological term, which has been used to describe processing noxious stimuli [10], the latter is a subjective feeling. In the present review, nociceptive reaction by noxious stimuli applied from the outside of the body are referred to as ‘nociception’, whereas ‘pain’ is the pain that is subjectively expressed by patients.
What is ANI and HFVI?
To properly interpret the results of monitoring instruments, it is important to understand the underlying technique and confounders. This section outlines the principles of ANI/HFVI.
The interval of R–R waves of the electrocardiogram (ECG) changes periodically due to the influence of the autonomic nervous system. This is called heart rate variability (HRV) and it has been studied for more than 50 years [11]. HRV can be observed by plotting the R–R interval of the ECG on a time series. When spectral analysis is performed on the periodic changes in HRV, HRV can be separated into a high frequency (HF) component that forms a peak in the frequency band of 0.15–0.4 (or 0.5) Hz and a low frequency (LF) component that forms a peak in 0.04–0.15 Hz [12]. The HF component reflects respiratory sinus arrhythmia, and it is known that the efferent vagal activity is a major contributor to the HF component [13].
ANI uses HRV to assess relative parasympathetic tone, and identifies R waves by 250 Hz digitized ECG. The obtained R–R samples are divided into 64 s moving windows and normalized by the following procedure [14].
First calculate the mean:
(M: mean value, n: the number of samples in the window, RRi: each R–R sample value).
Then calculate the norm value:
(N: norm value, n: the number of samples in window, RRi: each R–R sample value, M: mean value).
Then divide each resulting R–R sample by the norm value (N):
(RRi: each R–R sample value, M: mean value, N: norm value).
The mean-centered and normalized R–R series is automatically filtered by a fast wavelet transform, and as a result of the computation of the R–R series, only the HF component is extracted in real time [14, 15].
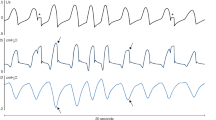
With changes in parasympathetic tone, the R–R series changes with breathing. When the parasympathetic tone is decreased, the effect of respiratory changes is reduced. As shown in Fig. 1, ANI divides the 64 s moving window into four 16 s sub-windows, and analyzes each sub-window. To eliminate the influence of changes in respiratory rate, local maxima are connected together as well as local minima, and the areas between the lower and upper envelopes [referred to as the area under the curve (AUC)] are analyzed [16]. The amplitude of the normalized and filtered R–R series ranges from 0 to 0.2 normalized unit [16]. The minimum AUC in each sub-window is defined as AUCmin, and their total is defined as AUCtotal; the maximum possible AUCtotal is 0.2 normalized unit × 64 s = 12.8 s. ANI calculates the percentage of the AUCtotal with a value between 0 and 100 using the following formula [14]:
The normalized and filtered R–R series are represented by solid lines. Each gray area (A1, A2, A3, and A4) is where the respiratory influence on the R–R series was measured. The upper panel is a high relative parasympathetic state with a sufficient antinociception state, and the lower panel is a low relative parasympathetic state with an insufficient antinociception state, in which the patient’s heart rate and blood pressure are increased. The respiratory cycle has a greater effect on the R–R series in the upper panel. (Modified and reproduced with permission) (Springer Nature; J Clin Monit Comput) [14]
The constants of α and β in the above formula are set to 5.1 and 1.2, respectively, by empirical determination in a general anesthesia dataset [17]. The average ANI for 2 and 4 min are continuously displayed on the monitor.
HFVI (Mdoloris Medical Systems) uses the same algorithm as ANI; therefore, ANI and HFVI are the same variables although they have different names. While ANI only is displayed on the ANI monitor initially, HFVI can be displayed along with other biometric information (e.g., electroencephalogram and percutaneous oxygen saturation) on the Root® (Masimo Corp.) monitor, using a dedicated module. Similar to ANI, HFVI also obtains ECG waveforms using two sensors positioned on the anterior chest (Fig. 2). HFVI was just commercialized in July 2022; therefore, no studies investigating HFVI have yet been published at the time of writing.
ANI for pain/nociception assessment
This section reviews randomized controlled trials and clinical studies with large numbers of subjects, with the aim of examining the usefulness and limitations of ANI in pain/nociception indicators. Due to the absence of absolute standard, clinical studies on nociception and pain are challenging, and each study considered the optimal timing to measure ANI and setting of outcome. When interpreting each study, the timing of ANI recording and the timing of nociception/pain set as the outcome are important.
Regarding the association between postoperative pain and ANI using a non-unit scale between 0 and 100 (100 indicates maximal relative parasympathetic tone), some observational studies have examined the association between ANI and NRS at post-anesthesia care unit (PACU) [18, 19]. A study investigating NRS and ANI in 200 post-surgery patients upon arrival at PACU reported a negative linear relationship between ANI and NRS (r2 = 0.41) and that the ANI thresholds to identify NRS > 3 and > 7 were 57 and 48, respectively [19]. In addition, Bosselli et al. investigated the association between ANI immediately before extubation and NRS immediately after arrival at the PACU in 200 patients who had undergone surgery under general anesthesia with inhalation and remifentanil [20]. They reported that if ANI < 50 before extubation is used as the threshold, pain with NRS > 3 can be predicted with a sensitivity of 86% and a specificity of 86% [area under the receiver-operating characteristic (ROC) curve (AUC): 0.89]. This result suggests that ANI may be able to predict postoperative pain in advance.
Several studies have compared ANI to other nociception monitoring techniques, that is, comparing ANI to SPI [21,22,23,24] and comparing ANI to pupillometry [25]. ANI and SPI are altered by nociceptive events under both inhalation [23] and propofol anesthesia [24]. It should be noted that SPI is the opposite of ANI, where 0 indicates complete analgesia and 100 indicates maximum nociception [26]. Charier et al. reported that pupillometry was more closely associated with a postoperative VAS score > 4 than ANI (AUC of 0.92 and 0.39, respectively) [25]; therefore, ANI does not appear to be superior to other nociception monitoring techniques. Further research is needed in this area.
The usefulness of ANI in children has also been investigated. In a study that analyzed ANI and hemodynamics for 5 min before the start of and for 5 min after the start of the surgical procedure under general anesthesia in children aged 2 to 12 years [27], it was reported that hemodynamics did not reflect the surgical stimulus while ANI did. In a study of children undergoing muscle biopsy under analgesia and light sedation, it was reported that there were negative correlations between the ANI and the FLACC scale [28]. Gall et al. examined ANI and FLACC scale in children younger than 7 years old in the recovery room who had undergone surgery or imaging studies (no surgical invasion) under general anesthesia [29]. They reported that the ANI cutoff value for predicting FLACC ≥ 4 was 56 (AUC, 0.94). The results of these studies suggest the utility of ANI in pediatric patients who may not be able to adequately describe their pain.
There are some interesting studies on the use of ANI in the intensive care unit (ICU). Chanques et al. investigated BPS and ANI during routine care at ICU [30]. As a result, instant-ANI (ANIi, an average calculated over a 64 s period) correlates with BPS (r = −0.30), and they revealed that BPS > 5 could be diagnosed with a sensitivity of 61.4%, a specificity of 77.4%, and a negative predictive value of 37.0% when ANIi 42.5 was used as a threshold. Another study was conducted in ICU in 21 patients with traumatic brain injury. It reported that there was a negative linear relationship between BPS and ANI (r2 = −0.469), and that it is possible to detect BPS ≥ 5 with a sensitivity of 73% and a specificity of 62% when the threshold of ANI is set to 50 [31]. Thus, in patients who cannot self-report pain, such as sedated patients, unconscious patients, or children, ANI can be useful in detecting the degree of pain and distress by setting the cutoff value to around 50, which was also suggested by the manufacturer.
Does ANI contribute to opioid-sparing anesthesia?
In recent years, opioid-sparing anesthesia with a multimodal approach in the perioperative period has been the common perception [32], and the same is true in the ICU [33]. This is because Enhanced Recovery After Surgery, or ERAS, which is aimed at good postoperative patient outcomes and early recovery, has been the focus of much attention [34]. The papers addressed in this section were extracted with the aim of scientifically examining the pros and cons of ANI’s contribution to opioid-sparing anesthesia.
Many studies have been conducted on whether using ANI during general anesthesia reduces intraoperative opioid consumption [15, 35]. Several studies have adjusted the dose of remifentanil or fentanyl to maintain the ANI around 50–70 during general anesthesia, and they revealed that ANI-guided management can reduce intraoperative opioid consumption without deteriorating postoperative outcomes [36,37,38,39,40]. Sabourdin et al. investigated ANI-guidance versus standard care in elective gynecologic surgery (n = 80) under target-controlled infusion of propofol and remifentanil [37]. They showed that intraoperative remifentanil consumption was lower in the ANI-guided group [4.4 vs 5.8 µg/kg/h, difference of −1.4; 95% confidence interval (95% CI) −2.6 to −0.2, p = 0.0026] with no difference in postoperative morphine consumption.
In contrast, Tribuddharat et al. conducted a prospective, randomized controlled study in 60 patients who underwent mastectomy under general inhalation anesthesia, and they reported that there was no difference in intraoperative fentanyl consumption, intraoperative heart rate and blood pressure, or postoperative pain score and morphine consumption between the ANI-guided group (received additional fentanyl when the ANI falls below 50) and the control group (received opioids based on hemodynamics such as tachycardia and increased blood pressure) [41]. In addition, Szental et al. reported that the rate of moderate/severe pain (VAS ≥ 50 mm) within the first postoperative hour was similar between the ANI-guided group (3–5 mg of morphine was added when the intraoperative ANI fell below 30–50) and the control group (anesthesiologist adjusted the drug without looking at the ANI) in 120 patients who had undergone laparoscopic cholecystectomy under general anesthesia (50.8% vs 45.0%, difference of −5.8%; 95% CI −23.7 to 12.1%, p = 0.58) [42]. Moreover, a meta-analysis that analyzed 10 studies of intraoperative opioid consumption and nociception monitoring (ANI, SPI, and pupillometry) revealed that nociception measurement-guided management reduces intraoperative opioids, while their subgroup analysis showed that intraoperative opioid consumption did not change between the ANI guidance and normal care groups [43]. Thus, no conclusion has been reached as to whether ANI contributes to opioid-sparing anesthesia. One reason for this is that the anesthetics used to maintain general anesthesia have different effects on HRV, and it has been pointed out that ANI may be more useful with propofol and remifentanil than with sevoflurane and fentanyl [44]. Interpretation of the results of studies on this topic also requires attention to the kinds of anesthetics used. Further studies on ANI with different anesthetics are needed.
General management with ANI monitoring
Studies on various ideas about how to utilize ANI for intraoperative management are being conducted. Jendoubi et al. investigated ANI in 100 patients who had undergone cesarean section under spinal anesthesia [45]. They reported that ANI at 3 min after spinal anesthesia declined significantly from baseline in the group with hypotension (systolic blood pressure decreased by 20% after spinal anesthesia or below 100 mmHg) compared with the group without hypotension. There may be little significance as ANI and blood pressure start to decline almost at the same time; however, the attempt to investigate the use of ANI as a predictor of hemodynamic changes is interesting. In addition, there have been several studies investigating changes in hemodynamics and ANI due to noxious stimuli during surgery in adult [14, 46,47,48,49] and pediatric patients [50, 51]. Jeanne et al. reported that in 27 patients who underwent total knee replacement under general anesthesia with propofol and sufentanil, the ANI threshold to detect hemodynamic reactivity was 63, with a sensitivity of 80% and a specificity of 88% (AUC: 0.92) [48]. In all of these studies, ANI seems to be more sensitive in assessing nociception than heart rate or blood pressure. Thus, monitoring ANI during general anesthesia may reduce adverse cardiovascular events and improve the safety of anesthesia [52].
In addition to changes in hemodynamics, there have been several studies exploring the potential of ANI. Xie et al. reported that in 98 patients who underwent painless abortion, ANI was significantly lower in the group with intraoperative movement (n = 42) than in the group without (n = 56) [53]. Le Guen et al. monitored ANI in 45 parturient women who underwent epidural anesthesia and reported that labor pains significantly reduced ANI. They also showed that the ANI cutoff value for predicting uterine contraction pain of VAS > 30 was 49 [54]. Furthermore, there are studies that have used ANI to determine the effectiveness of regional anesthesia [35, 55]. Migeon et al. analyzed 39 successful and 19 unsuccessful regional anesthesia (defined as 10 bpm increase in heart rate within 2 min from the start of surgery) in children with peripheral nerve block or central neuraxial block. They described that ANI < 51 can identify regional anesthesia failure with a sensitivity of 78% and a specificity of 62% (AUC: 0.747) [55]. Thus, the association between various outcomes and ANI as well as opioid titration and hemodynamic changes detected during general anesthesia has been studied. However, the number of studies is small; therefore, further research in various population groups is needed to make the most of ANI. Compared to ANI, the newly named HFVI may be more easily understood not as a pain index but as values that indicate relative autonomic balance. Studies that apply HFVI to the general management of patients may increase in the future.
Limitations of ANI
ANI utilizes respiratory fluctuations in the ECG. Therefore, it cannot be used in patients with severe arrhythmias, arterial fibrillation, implanted pacemaker, or cardiopulmonary bypass, or in patients treated with antimuscarinic drugs, etc. [15]. In addition, some studies have reported that ANI values in patients differ when under general anesthesia and when in a conscious state [56]; thus, ANI in an awake state is difficult to interpret [57]. Baroni et al. performed a meta-analysis of nine studies that assessed ANI and self-reported measures in conscious patients [58]. They found a weak negative correlation between ANI and NRS in the post-anesthetic recovery room (r = −0.0984; 95% CI −0.397 to 0.220, I2 = 95.82%). They described that one possible reason for the variation in ANI in a conscious state is the influence of emotion. Jess et al. investigated ANI in conscious volunteers when receiving four stimuli of expected/unexpected electrical pain and expected nonpainful/sham stimuli, and reported that ANI was changed by stress and emotion [59]. Furthermore, other studies were performed on changes in ANI with emotional changes elicited by music [6] and with negative emotional stimuli through videos [17]. Both studies revealed that ANI was a good indicator of parasympathetic changes related to the emotional state. Although ANI may be an objective indicator of emotional changes, at present, it is not possible to assess postoperative nociception/pain by ANI alone in conscious patients.
In addition, regarding the effects of drugs on ANI, ephedrine has been reported to affect ANI [60]. Meanwhile, it was shown that ANI was less affected by esmolol in septic shock piglet model [61]. Although ANI may be affected by anesthetics such as those mentioned above [44], Bollag et al. reported that intravenous administration of ketamine 0.5 mg/kg had no effect on ANI [62]. However, it should be noted that the effect of higher doses of ketamine on ANI has not been investigated yet. It can be said that when interpreting ANI, it is necessary to refer to the clinical situation and trends of ANI, even during general anesthesia. Despite advances in nociception monitoring technology and availability, limitations of HRV-derived indicators presently override their benefits in routine anesthesia care at this time. Thus, future research is needed to understand the implications of ANI/HFVI changes and their value itself in a situation where the ANI/HFVI assessment of pain is unreliable.
Outlooks of ANI/HFVI
In this section, we will discuss how ANI/HFVI can be used other than to monitor nociception/pain by reviewing some of the literature. Regarding the potential of ANI, Anderson et al. described that ANI-based analgesia has the potential to be good for individualized titration of anesthesia management [63], and trials of ANI-based goal-directed analgesia are currently underway [64]. If ANI/HFVI studies are accumulated and their validity is confirmed, ANI-based administration may be realized [37]. A machine learning-based method combining ANI and hemodynamic monitoring has already been investigated with continuous administration of remifentanil [65]. These trends indicate the promising development of automated anesthesia/general management system by combining artificial intelligence and ANI/HFVI.
ANI at the end of life has also been studied. Bauschert et al. investigated simultaneous clinical and ANI evaluations in a palliative care setting, and reported that they were concordant in 77.58% of episodes [66]. This result suggests the possible use of ANI in the care that is performed based on staff’s experience and intuition such as the end-of-life care for non-communicative patients.
The impact of ANI on prognosis is also an interesting area. A study of 14 patients with severe coronavirus disease 2019 who required mechanical ventilation reported higher ANI and IL-6 in the non-survivor group [67]. They found that parasympathetic dominance due to sympathetic depletion may lead to a poor prognosis. Thus, further studies on the association between prognosis and ANI/HFVI in various population groups in ICU are necessary. In addition, a study monitoring ANI in one-day surgery reported that the group with ANI ≥ 50 for at least 60% of the time they were under anesthesia had a significantly shorter hospital stay than those with ANI < 50 [165 min (118–212) vs 186.5 min (119–254), p = 0.0425] [68]. As such, investigation of association between comprehensive outcomes following day surgery/anesthesia and ANI are also interesting. Such studies may be easier to understand by renaming HFVI from ANI.
Conclusion
In this article, we reviewed key papers on ANI that have been published in the last decade, introduced HFVI, and discussed the outlooks of ANI/HFVI. At present, ANI has a certain usefulness as a nociception/pain monitor during or immediately after general anesthesia in patients undergoing surgery, and in severely ill patients in the ICU. However, it is currently not possible to evaluate nociception/pain using ANI alone, especially in the awake state. With the commercialization of HFVI, it is expected that the attention of ANI/HFVI will increase. ANI/HFVI has the potential to become a mainstream monitoring method in a next era, not just for nociception/pain monitoring, and further study of its use not only in the operative room and ICU, but in other fields is awaited.
Data availability
Data are available upon reasonable request.
References
Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161:1976–82.
Ledowski T. Objective monitoring of nociception: a review of current commercial solutions. Br J Anaesth. 2019;123:e312–21.
von Dincklage F, Ledowski T. Monitoring of nociception: is more always more? Br J Anaesth. 2021;126:352–4.
Crellin DJ, Harrison D, Santamaria N, Huque H, Babl FE. The psychometric properties of the FLACC scale used to assess procedural pain. J Pain. 2018;19:862–72.
Sabourdin N, Constant I. Monitoring of analgesia level during general anesthesia in children. Curr Opin Anaesthesiol. 2022;35:367–73.
Ledowski T, Pascoe E, Ang B, Schmarbeck T, Clarke MW, Fuller C, Kapoor V. Monitoring of intra-operative nociception: skin conductance and surgical stress index versus stress hormone plasma levels. Anaesthesia. 2010;65:1001–6.
Neice AE, Behrends M, Bokoch MP, Seligman KM, Conrad NM, Larson MD. Prediction of opioid analgesic efficacy by measurement of pupillary unrest. Anesth Analg. 2017;124:915–21.
Ledowski T, Burke J, Hruby J. Surgical pleth index: prediction of postoperative pain and influence of arousal. Br J Anaesth. 2016;117:371–4.
Martini CH, Boon M, Broens SJ, Hekkelman EF, Oudhoff LA, Buddeke AW, Dahan A. Ability of the nociception level, a multiparameter composite of autonomic signals, to detect noxious stimuli during propofol-remifentanil anesthesia. Anesthesiology. 2015;123:524–34.
Loeser JD, Treede RD. The Kyoto protocol of IASP basic pain terminology. Pain. 2008;137:473–7.
Sayers BM. Analysis of heart rate variability. Ergonomics. 1973;16:17–32.
Jeanne M, Logier R, De Jonckheere J, Tavernier B. Validation of a graphic measurement of heart rate variability to assess analgesia/nociception balance during general anesthesia. Annu Int Conf IEEE Eng Med Biol Soc. 2009;2009:1840–3.
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65.
Jeanne M, Clément C, De Jonckheere J, Logier R, Tavernier B. Variations of the analgesia nociception index during general anaesthesia for laparoscopic abdominal surgery. J Clin Monit Comput. 2012;26:289–94.
Daccache G, Jeanne M, Fletcher D. The analgesia nociception index: tailoring opioid administration. Anesth Analg. 2017;125:15–7.
Abdullayev R, Yildirim E, Celik B, Topcu SL. Analgesia nociception index: heart rate variability analysis of emotional status. Cureus. 2019;11: e4365.
De Jonckheere J, Rommel D, Nandrino JL, Jeanne M, Logier R. Heart rate variability analysis as an index of emotion regulation processes: interest of the analgesia nociception index (ANI). Annu Int Conf IEEE Eng Med Biol Soc. 2012;2012:3432–5.
Ledowski T, Tiong WS, Lee C, Wong B, Fiori T, Parker N. Analgesia nociception index: evaluation as a new parameter for acute postoperative pain. Br J Anaesth. 2013;111:627–9.
Boselli E, Daniela-Ionescu M, Bégou G, Bouvet L, Dabouz R, Magnin C, Allaouchiche B. Prospective observational study of the non-invasive assessment of immediate postoperative pain using the analgesia/nociception index (ANI). Br J Anaesth. 2013;111:453–9.
Boselli E, Bouvet L, Bégou G, Dabouz R, Davidson J, Deloste JY, Rahali N, Zadam A, Allaouchiche B. Prediction of immediate postoperative pain using the analgesia/nociception index: a prospective observational study. Br J Anaesth. 2014;112:715–21.
Choi BM, Shin H, Lee JH, Bang JY, Lee EK, Noh GJ. Performance of the surgical pleth index and analgesia nociception index in healthy volunteers and parturients. Front Physiol. 2021;12: 554026.
Dostalova V, Schreiberova J, Bartos M, Kukralova L, Dostal P. Surgical pleth index and analgesia nociception index for intraoperative analgesia in patients undergoing neurosurgical spinal procedures: a comparative randomized study. Minerva Anestesiol. 2019;85:1265–72.
Gruenewald M, Herz J, Schoenherr T, Thee C, Steinfath M, Bein B. Measurement of the nociceptive balance by analgesia nociception index and surgical pleth index during sevoflurane-remifentanil anesthesia. Minerva Anestesiol. 2015;81:480–9.
Gruenewald M, Ilies C, Herz J, Schoenherr T, Fudickar A, Höcker J, Bein B. Influence of nociceptive stimulation on analgesia nociception index (ANI) during propofol-remifentanil anaesthesia. Br J Anaesth. 2013;110:1024–30.
Charier D, Vogler MC, Zantour D, Pichot V, Martins-Baltar A, Courbon M, Roche F, Vassal F, Molliex S. Assessing pain in the postoperative period: analgesia nociception index™ versus pupillometry. Br J Anaesth. 2019;123:e322–7.
Struys MM, Vanpeteghem C, Huiku M, Uutela K, Blyaert NB, Mortier EP. Changes in a surgical stress index in response to standardized pain stimuli during propofol-remifentanil infusion. Br J Anaesth. 2007;99:359–67.
Julien-Marsollier F, Rachdi K, Caballero MJ, Ayanmanesh F, Vacher T, Horlin AL, Skhiri A, Brasher C, Michelet D, Dahmani S. Evaluation of the analgesia nociception index for monitoring intraoperative analgesia in children. Br J Anaesth. 2018;121:462–8.
Avez-Couturier J, De Jonckheere J, Jeanne M, Vallée L, Cuisset JM, Logier R. Assessment of procedural pain in children using analgesia nociception index: a pilot study. Clin J Pain. 2016;32:1100–4.
Gall O, Champigneulle B, Schweitzer B, Deram T, Maupain O, Montmayeur Verchere J, Orliaguet G. Postoperative pain assessment in children: a pilot study of the usefulness of the analgesia nociception index. Br J Anaesth. 2015;115:890–5.
Chanques G, Tarri T, Ride A, Prades A, De Jong A, Carr J, Molinari N, Jaber S. Analgesia nociception index for the assessment of pain in critically ill patients: a diagnostic accuracy study. Br J Anaesth. 2017;119:812–20.
Jendoubi A, Abbes A, Ghedira S, Houissa M. Pain measurement in mechanically ventilated patients with traumatic brain injury: behavioral pain tools versus analgesia nociception index. Indian J Crit Care Med. 2017;21:585–8.
Shanthanna H, Ladha KS, Kehlet H, Joshi GP. Perioperative opioid administration. Anesthesiology. 2021;134:645–59.
Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B, Balas MC, van den Boogaard M, Bosma KJ, Brummel NE, Chanques G, Denehy L, Drouot X, Fraser GL, Harris JE, Joffe AM, Kho ME, Kress JP, Lanphere JA, McKinley S, Neufeld KJ, Pisani MA, Payen JF, Pun BT, Puntillo KA, Riker RR, Robinson BRH, Shehabi Y, Szumita PM, Winkelman C, Centofanti JE, Price C, Nikayin S, Misak CJ, Flood PD, Kiedrowski K, Alhazzani W. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–73.
Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292–8.
Theerth KA, Sriganesh K, Reddy KM, Chakrabarti D, Umamaheswara Rao GS. Analgesia nociception index-guided intraoperative fentanyl consumption and postoperative analgesia in patients receiving scalp block versus incision-site infiltration for craniotomy. Minerva Anestesiol. 2018;84:1361–8.
Soral M, Altun GT, Dinçer PÇ, Arslantaş MK, Aykaç Z. Effectiveness of the analgesia nociception index monitoring in patients who undergo colonoscopy with sedo-analgesia. Turk J Anaesthesiol Reanim. 2020;48:50–7.
Sabourdin N, Burey J, Tuffet S, Thomin A, Rousseau A, Al-Hawari M, Taconet C, Louvet N, Constant I. Analgesia nociception index-guided remifentanil versus standard care during propofol anesthesia: a randomized controlled trial. J Clin Med. 2022;11:333.
Dundar N, Kus A, Gurkan Y, Toker K, Solak M. Analgesia nociception index (ani) monitoring in patients with thoracic paravertebral block: a randomized controlled study. J Clin Monit Comput. 2018;32:481–6.
Le Gall L, David A, Carles P, Leuillet S, Chastel B, Fleureau C, Dewitte A, Ouattara A. Benefits of intraoperative analgesia guided by the analgesia nociception index (ANI) in bariatric surgery: an unmatched case-control study. Anaesth Crit Care Pain Med. 2019;38:35–9.
Upton HD, Ludbrook GL, Wing A, Sleigh JW. Intraoperative “analgesia nociception index”-guided fentanyl administration during sevoflurane anesthesia in lumbar discectomy and laminectomy: a randomized clinical trial. Anesth Analg. 2017;125:81–90.
Tribuddharat S, Sathitkarnmanee T, Sukhong P, Thananun M, Promkhote P, Nonlhaopol D. Comparative study of analgesia nociception index (ANI) vs standard pharmacokinetic pattern for guiding intraoperative fentanyl administration among mastectomy patients. BMC Anesthesiol. 2021;21:50.
Szental JA, Webb A, Weeraratne C, Campbell A, Sivakumar H, Leong S. Postoperative pain after laparoscopic cholecystectomy is not reduced by intraoperative analgesia guided by analgesia nociception index (ANI®) monitoring: a randomized clinical trial. Br J Anaesth. 2015;114:640–5.
Jiao Y, He B, Tong X, Xia R, Zhang C, Shi X. Intraoperative monitoring of nociception for opioid administration: a meta-analysis of randomized controlled trials. Minerva Anestesiol. 2019;85:522–30.
Boselli E, Jeanne M. Analgesia/nociception index for the assessment of acute postoperative pain. Br J Anaesth. 2014;112:936–7.
Jendoubi A, Khalloufi A, Nasri O, Abbes A, Ghedira S, Houissa M. Analgesia nociception index as a tool to predict hypotension after spinal anaesthesia for elective caesarean section. J Obstet Gynaecol. 2021;41:193–9.
Ledowski T, Averhoff L, Tiong WS, Lee C. Analgesia nociception index (ANI) to predict intraoperative haemodynamic changes: results of a pilot investigation. Acta Anaesthesiol Scand. 2014;58:74–9.
Boselli E, Logier R, Bouvet L, Allaouchiche B. Prediction of hemodynamic reactivity using dynamic variations of analgesia/nociception index (∆ANI). J Clin Monit Comput. 2016;30:977–84.
Jeanne M, Delecroix M, De Jonckheere J, Keribedj A, Logier R, Tavernier B. Variations of the analgesia nociception index during propofol anesthesia for total knee replacement. Clin J Pain. 2014;30:1084–8.
Boselli E, Bouvet L, Bégou G, Torkmani S, Allaouchiche B. Prediction of hemodynamic reactivity during total intravenous anesthesia for suspension laryngoscopy using analgesia/nociception index (ANI): a prospective observational study. Minerva Anestesiol. 2015;81:288–97.
Sabourdin N, Arnaout M, Louvet N, Guye ML, Piana F, Constant I. Pain monitoring in anesthetized children: first assessment of skin conductance and analgesia-nociception index at different infusion rates of remifentanil. Paediatr Anaesth. 2013;23:149–55.
Weber F, Geerts NJE, Roeleveld HG, Warmenhoven AT, Liebrand CA. The predictive value of the heart rate variability-derived analgesia nociception index in children anaesthetized with sevoflurane: an observational pilot study. Eur J Pain. 2018;22:1597–605.
De Jonckheere J, Delecroix M, Jeanne M, Keribedj A, Couturier N, Logier R. Automated analgesic drugs delivery guided by vagal tone evaluation: interest of the analgesia nociception index (ANI). Annu Int Conf IEEE Eng Med Biol Soc. 2013;2013:1952–5.
Xie H, Chen W, Liu J, Li J, Li S. Changes of analgesia/nociception index under different surgical steps in abortion under general anesthesia: a prospective clinical study. J Clin Anesth. 2020;66: 109898.
Le Guen M, Jeanne M, Sievert K, Al Moubarik M, Chazot T, Laloë PA, Dreyfus JF, Fischler M. The analgesia nociception index: a pilot study to evaluation of a new pain parameter during labor. Int J Obstet Anesth. 2012;21:146–51.
Migeon A, Desgranges FP, Chassard D, Blaise BJ, De Queiroz M, Stewart A, Cejka JC, Combet S, Rhondali O. Pupillary reflex dilatation and analgesia nociception index monitoring to assess the effectiveness of regional anesthesia in children anesthetised with sevoflurane. Paediatr Anaesth. 2013;23:1160–5.
Lee JH, Choi BM, Jung YR, Lee YH, Bang JY, Noh GJ. Evaluation of surgical pleth index and analgesia nociception index as surrogate pain measures in conscious postoperative patients: an observational study. J Clin Monit Comput. 2020;34:1087–93.
Issa R, Julien M, Décary E, Verdonck O, Fortier LP, Drolet P, Richebé P. Evaluation of the analgesia nociception index (ANI) in healthy awake volunteers. Can J Anaesth. 2017;64:828–35.
Baroni DA, Abreu LG, Paiva SM, Costa LR. Comparison between analgesia nociception index (ANI) and self-reported measures for diagnosing pain in conscious individuals: a systematic review and meta-analysis. Sci Rep. 2022;12:2862.
Jess G, Pogatzki-Zahn EM, Zahn PK, Meyer-Frießem CH. Monitoring heart rate variability to assess experimentally induced pain using the analgesia nociception index: A randomised volunteer study. Eur J Anaesthesiol. 2016;33:118–25.
Graça R, Lobo FA. Analgesia nociception index (ANI) and ephedrine: a dangerous liasion. J Clin Monit Comput. 2021;35:953–4.
Boselli E, Jacquet-Lagrèze M, Ayoub JY, Bouvet L, Dauwalder O, Mansour C, Margez T, Paquet C, Restagno D, Allaouchiche B, Bonnet-Garin JM, Junot S. Effects of esmolol on systemic hemodynamics and heart rate variability measured using the analgesia/nociception index in resuscitated piglets with Pseudomonas aeruginosa septic shock. J Vet Emerg Crit Care (San Antonio). 2018;28:447–56.
Bollag L, Ortner CM, Jelacic S, Rivat C, Landau R, Richebé P. The effects of low-dose ketamine on the analgesia nociception index (ANI) measured with the novel PhysioDoloris™ analgesia monitor: a pilot study. J Clin Monit Comput. 2015;29:291–5.
Anderson TA, Segaran JR, Toda C, Sabouri AS, De Jonckheere J. High-frequency heart rate variability index: a prospective, observational trial assessing utility as a marker for the balance between analgesia and nociception under general anesthesia. Anesth Analg. 2020;130:1045–53.
Michalot A, Bazin JÉ, Richebé P, Allaouchiche B, Boselli E. Effect of GOAL-Directed ANalgesia using ANI (Analgesia/Nociception Index) during general anesthesia on immediate postoperative pain and intraoperative hemodynamics in adult patients (GOALDAN study): a study protocol for randomized, controlled, multicenter trial. Trials. 2022;23:353.
Gonzalez-Cava JM, Arnay R, León A, Martín M, Reboso JA, Calvo-Rolle JL, Mendez-Perez JA. Machine learning based method for the evaluation of the analgesia nociception index in the assessment of general anesthesia. Comput Biol Med. 2020;118: 103645.
Bauschert L, Prod’homme C, Pierrat M, Chevalier L, Lesaffre H, Touzet L. End-of-life comfort evaluation, is clinic enough? A retrospective cohort study of combined comfort evaluation with analgesia/nociception index and clinic in non-communicative patients. J Palliat Care. 2021. https://doi.org/10.1177/08258597211063687.
Aragón-Benedí C, Oliver-Forniés P, Galluccio F, Yamak Altinpulluk E, Ergonenc T, El Sayed AA, Salazar C, Fajardo-Pérez M. Is the heart rate variability monitoring using the analgesia nociception index a predictor of illness severity and mortality in critically ill patients with COVID-19? A pilot study. PLoS ONE. 2021;16: e0249128.
Ramos-Luengo A, Gardeta Pallarés A, Asensio MF. Usefulness of ANI (analgesia nociception index) monitoring for outpatient saphenectomy surgery outcomes: an observational study. J Clin Monit Comput. 2021;35:491–7.
Acknowledgements
The authors would like to thank Dr. M. Jeanne and Springer Nature for giving us permission to use Fig. 1. The authors are also deeply grateful to Masayuki Shindo and Rieko Yamato of Masimo Japan Corp. (Tokyo, Japan) for confirming that the technical details in the manuscript are correct. In addition, we would like to thank Shuhei Agari of HeiwaBussan Co., Ltd (Tokyo, Japan) for confirming that the technical details in the manuscript are correct. (Shindo, Yamato, and Agari were not involved in the writing of the manuscript.) Finally, the authors would like to thank the Scientific English Editing Section of Fukushima Medical University for their work on this manuscript.
Funding
The authors have no sources of funding to declare for this manuscript.
Author information
Authors and Affiliations
Contributions
KY prepared the manuscript. SO and SI helped to draft the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Patient consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yoshida, K., Obara, S. & Inoue, S. Analgesia nociception index and high frequency variability index: promising indicators of relative parasympathetic tone. J Anesth 37, 130–137 (2023). https://doi.org/10.1007/s00540-022-03126-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-022-03126-8