Abstract
In cardiac surgery, use of the antifibrinolytic agent tranexamic acid (TXA) and acute perioperative stroke are both associated with convulsive seizures. We hypothesized that an older (preoperative) stroke increases the risk of TXA-associated seizures as well. To test this hypothesis, we retrospectively analyzed data from 16,110 patients who had undergone open-heart valvular surgery at our institution between 2009 and 2020. The dosing of TXA was moderate. Use of TXA and a history of stroke were both independently associated with convulsive seizure with an adjusted odds ratio (OR) of 2.40 (95%CI: 1.71–3.37) and 1.79 (95%CI: 1.27–2.54), respectively. Compared to patients without TXA administration, the adjusted OR of experiencing a seizure in TXA patients without a history of stroke was 2.44 (95%CI: 1.71–3.46) and in patients receiving TXA with a history of stroke 4.30 (95%CI: 2.65–6.99). However, there was no significant interaction between TXA use and preoperative stroke on convulsive seizures (P = 0.77). Compared to patients without seizure, for patients with seizure, the inverse probability-weighted ORs of in-hospital mortality and 30-day mortality were 3.58 (95%CI: 2.20–5.83) and 4.04 (95%CI: 2.34–6.98), respectively. We conclude that, in patients undergoing open-heart surgery, a history of stroke is independently associated with convulsive seizures but is not a contraindication for TXA use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In cardiac surgery, the antifibrinolytic agent tranexamic acid (TXA) has been associated with convulsive seizures [1]. The underlying pharmacologic mechanism is considered to be a disinhibition of cerebral glycine and gamma-aminobutyric acid receptors [2]. To date, increasing TXA dose and open-heart surgery are the only parameters that have been clearly established as underlying risk factors of convulsive seizures [1,2,3]. In the large randomized, placebo-controlled ATACAS (Aspirin and Tranexamic Acid for Coronary Artery Surgery) trial that included patients undergoing isolated valvular or combined valvular and coronary artery bypass grafting (CABG) surgery, use of TXA (100 mg and 50 mg per kg body weight in 75% and 25% of trial participants, respectively) was associated with a higher risk of postoperative seizures than in the placebo group [3]. In addition, a strong positive relationship between a perioperative stroke and a seizure was observed [3]. We therefore hypothesized that a history of stroke may also be a critical risk factor for TXA-associated seizures. To test this hypothesis, we analyzed data from our high-volume center over the last 10 years.
Methods
Patient selection and anesthesia technique
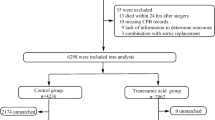
We performed a retrospective analysis in patients who had undergone either isolated valvular or combined valvular and CABG open-heart surgery at our institution between July 2009 and September 2020. Patients with concomitant aortic surgery were excluded. After further exclusion of 47 patients with missing pre- or perioperative data, a total of 16,110 patients could ultimately be analyzed (Table 1). Anesthesia was introduced with etomidate, rocuronium and sufentanil and maintained with a continuous infusion of remifentanil (0.5–1 µg/kg/min) and vaporization of sevoflurane. After closure of the chest, sevoflurane was replaced by a continuous infusion of propofol (2–4 mg/kg/h). The decision to give TXA was agreed between the surgeon and the anesthetist based on the degree of microvascular bleeding before heparinization. TXA was given with a bolus of 1 g after heparinization, followed by an infusion of 0.2 g/h until the end of cardiopulmonary bypass. In the priming volume of the cardiopulmonary bypass system, 0.5 g of TXA was added. For antibiotic prophylaxis, 2 g of cefazolin was given after the induction of anesthesia, and thereafter at intervals of 3 h.
Data collection and study design
We assessed 19 preoperative patient characteristics (Table 1) and three outcome parameters (seizure, in-hospital mortality, 30-day mortality), in addition to the two parameters history of stroke and use of TXA. A seizure was considered to have occurred in the case of sudden clonic movement of the patient and was verified by a computer tomography scan. For data analysis, the study cohort was divided into four subgroups: patients with or without preoperative stroke and with or without perioperative TXA administration.
Statistical analysis
Categorical variables are summarized as percentages and number of observations. Preoperative continuous variables are presented as median with 25th and 75th percentiles. We used the Mann–Whitney U test and Fisher’s exact test to assess group differences in continuous and categorical variables where appropriate. We performed univariate and multivariable-adjusted binary logistic regression analysis to assess the association of study group with perioperative seizure risk (see Supplemental Material). Data are presented as odds ratio (OR) with 95% confidence interval (CI). A sample size calculation with Bonferroni correction for multiplicity revealed that with 500 patients in the non-stroke, non-TXA group and 500 patients in the stroke, TXA group, there will be a 90% chance of detecting a significant difference at a two-sided 0.0125 significance level. This assumes incidence rates of 1% and 5%, respectively. We also tested for interaction (based on the Wald test for interaction term) between TXA administration and preoperative stroke with adjustment of covariates. To assess associations of seizures with in-hospital and 30-day mortality, we applied the method of inverse probability weighting (see Supplemental Material). P values < 0.05 were considered statistically significant. We used the statistical software package IBM SPSS, version 27 (IBM Corp, Armonk, NY, USA), to perform the analyses.
Results
Use of TXA and a history of stroke were both independently associated with convulsive seizure (Supplemental Table 1). The four groups of patients with or without preoperative stroke and with or without perioperative TXA administration differed substantially with respect to most baseline characteristics assessed, with the exception of age and smoking status (Table 1). Compared to patients without TXA administration, the adjusted OR of experiencing a seizure in TXA patients without a history of stroke was 2.44 (95%CI: 1.71–3.46) and in patients receiving TXA with a history of stroke 4.30 (95%CI: 2.65–6.99) (Table 2). There was, however, no significant interaction between TXA use and preoperative stroke on convulsive seizures (P = 0.77).
In-hospital mortality and 30-day mortality were 3.4% (n = 547) and 3.5% (n = 569), respectively. Compared with patients without seizure, for patients with seizure, inverse probability-weighted in-hospital mortality and 30-day mortality were 3.58 (95%CI: 2.20–5.83) and 4.04 (95%CI: 2.34–6.98), respectively.
Discussion
Our results indicate that a history of stroke and use of TXA are both independently associated with an increased risk of seizure in patients undergoing valvular open-heart surgery. However, our data do not support the hypothesis of a significant interaction of both parameters on the risk of seizure.
It is well known that, in open-heart surgery, TXA dose-dependently increases the risk of convulsive seizure [1, 3]. This is attributed to toxic TXA concentrations (≥ 200 µM) in the cerebral fluid. The underlying cause is considered to be an increased permeability of the blood–brain barrier due to systemic inflammation and its jeopardization via micro-air embolism following open-heart surgery or cerebral injury [2]. With respect to acute stroke, dysfunction of the blood–brain barrier is a prominent characteristic, and it is unclear whether complete restoration occurs [4]. Therefore, a history of stroke may increase the risk of seizure by persistent blood–brain barrier impairment, particularly in the aged brain [4]. However, the concept of an impaired blood–brain barrier has been challenged by the results of two recent large randomized placebo-controlled trials in acute traumatic brain injury and acute intracerebral hemorrhage [5, 6]. In both trials, the dosing of TXA was almost comparable to our protocol. The CRASH-3 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage) trial allocated 12,737 patients with traumatic brain injury to TXA or matching placebo [5]. The risk of developing seizures did not differ significantly between groups [5]. The TICH-2 (Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage) trial allocated 2325 patients with acute spontaneous intracerebral hemorrhage to TXA or matching placebo [6]. The incidence of seizures in both groups was 7% [6]. These data show that the mechanisms leading to toxic TXA concentrations in cardiac surgery and potentially promoting stroke-related convulsive seizures require further research. The results of the two aforementioned studies are also in line with our finding that there was no significant interaction between a history of stroke and TXA administration on seizures. Obviously, there is no similar mechanism of how both parameters may influence the risk of seizures.
It should be emphasized that our results of a higher short-term mortality risk in patients with seizures than in patients without seizures are in line with earlier findings [3].
Our investigation has several limitations that have to be addressed. First, the retrospective study design prevents us from assuming causal relationships between risk factors and outcomes. Second, the small numbers of patients in the two groups with a history of stroke may have reduced statistical power for detecting significant interactions between use of TXA and a history of stroke on convulsive seizures. Third, we cannot rule out that study results are biased by unexplained confounding. Finally, the propensity score for calculating inverse probability weighting was solely based on preoperative covariate data and did not include perioperative parameters.
In summary, our data indicate that, in patients undergoing open-heart surgery, a history of stroke is independently associated with convulsive seizures. Since there was no significant interaction between TXA use and preoperative stroke on convulsive seizures, a history of stroke is not a contraindication for TXA use.
Change history
28 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00540-021-02936-6
References
Lin Z, Xiaoyi Z. Tranexamic acid-associated seizures: a meta-analysis. Seizure. 2016;36:70–3.
Lecker I, Wang DS, Whissell PD, Avramescu S, Mazer CD, Orser BA. Tranexamic acid associated seizures: causes and treatment. Ann Neurol. 2016;79:18–26.
Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T, et al. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2017;376:136–48.
Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, Keep RF, Shi Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018;163–164:144–71.
CRASH-3 trial collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. 2019;394:1713–23.
Sprigg N, Flaherty K, Appleton JP, Al-Shahi Salman R, Bereczki D, Beridze M. Tranexamic acid to improve functional status in adults with spontaneous intracerebral haemorrhage: the TICH-2 RCT. Health Technol Assess. 2019;23:1–48.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. NH collected data. AZ performed the statistical analysis. NH, AZ and AK wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to the last name of corresponding author was published incorrectly and corrected in this version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hulde, N., Zittermann, A., Deutsch, MA. et al. Associations of preoperative stroke and tranexamic acid administration with convulsive seizures in valvular open-heart surgery. J Anesth 35, 451–454 (2021). https://doi.org/10.1007/s00540-021-02924-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-021-02924-w