Abstract
Background
We compared the pharmacokinetics of levobupivacaine when administered intraperitoneally, subcutaneously, and intravenously in an anesthetized rat model, to estimate the toxicity risk of a local anesthetic when absorbed from the peritoneum.
Methods
Thirty-two rats were anesthetized with sevoflurane. In Experiment 1, we administered 5.0 mg/kg of levobupivacaine intraperitoneally (IP) (n = 7), subcutaneously (SC) (n = 6), or intravenously (IV) (n = 6). In Experiment 2, we administered 2.5 mg/kg of levobupivacaine IP (n = 7) or SC (n = 6). Data are shown as median [range] of Experiment 1.
Results
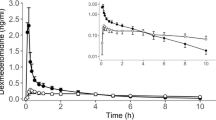
In either of experiments, the time to reach maximum plasma concentration of levobupivacaine was shorter in the IP group than in the SC group (IP: 2 [2–5] min; SC: 5 [2–10] min; P = 0.04), and the maximum concentration of levobupivacaine did not differ between the IP and SC groups (IP: 0.45 [0.05–0.67] µg/mL; SC: 0.47 [0.21–0.62] µg/mL; P = 0.90). The area under the curve from time 0 to 120 min after levobupivacaine administration was significantly higher in the SC group than in the IP group in both experiments (IP: 0.29 [0.10–0.54] mg h/L; SC: 0.78 [0.39–0.98] mg h/L; P = 0.04).
Conclusion
Levobupivacaine is rapidly absorbed following IP administration, but its maximum plasma concentration within 2 h following IP administration is no statistical difference as that following SC administration. On the other hand, when levobupivacaine is given subcutaneously, Tmax can exceed 1 h, so we need to be aware of local anesthetic toxicity during this period.


Similar content being viewed by others
References
Sites BD, Taenzer AH, Herrick MD, Gilloon C, Antonakakis J, Richins J, Beach ML. Incidence of local anesthetic systemic toxicity and postoperative neurologic symptoms associated with 12,668 ultrasound-guided nerve blocks: an analysis from a prospective clinical registry. Reg Anesth Pain Med. 2012;37:478–82.
Barrington MJ, Watts SA, Gledhill SR, Thomas RD, Said SA, Suyder GL, Tay VS, Jamrozik K. Preliminary results of the Australasian Regional Anaesthesia Collaboration: a prospective audit of more than 7000 peripheral nerve and plexus blocks for neurologic and other complications. Reg Anesth Pain Med. 2009;34:534–41.
Aydin G. Unexpected local anesthesia toxicity during the ultrasonography-guided peripheral nerve block. J Clin Anesth. 2018;50:26.
Rosenberg PH, Veering BT, Urmey WF. Maximum recommended doses of local anesthetics: a multifactorial concept. Reg Anesth Pain Med. 2004;29:564–75.
Griffiths JD, Barron FA, Grant S, Bjorksten AR, Hebbard P, Royse CF. Plasma ropivacaine concentrations after ultrasound-guided transversus abdominis plane block. Br J Anaesth. 2010;105:853–6.
Rafi AN. Abdominal field block: a new approach via the lumbar triangle. Anaesthesia. 2001;56:1024–6.
McDermott G, Korba E, Mata U, Jaigirdar M, Narayanan N, Boylan J, Conlon N. Should we stop doing blind transversus abdominis plane blocks? Br J Anaesth. 2012;108:499–502.
Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10.
Lim CK, Lord G. Current developments in LC-MS for pharmaceutical analysis. Biol Pharm Bull. 2002;25:547–57.
Yamaoka K, Nakagawa T, Uno T. Statistical moments in pharmacokinetics. J Pharmacokinet Biopharm. 1978;6:547–58.
Lukas G, Brindle SD, Greengard P. The route of absorption of intraperitoneally administered compounds. J Pharmacol Exp Ther. 1971;178:562–4.
Myers CE, Collins JM. Pharmacology of intraperitoneal chemotherapy. Cancer Investig. 1983;1:395–407.
Narchi P, Benhamou D, Bouaziz H, Fernandez H, Mazoit JX. Plasma concentrations of local anaesthetics following intraperitoneal administration during laparoscopy. Eur J Clin Pharmacol. 1992;42:223–5.
Pond SM, Tozer TN. First-pass elimination. Basic concepts and clinical consequences. Clin Pharmacokinet. 1984;9:1–25.
Williams R, White H. The greater omentum: its applicability to cancer surgery and cancer therapy. Curr Probl Surg. 1986;23:789–865.
Surbone A, Myers CE. Principles and practice of intraperitoneal therapy. Antibiot Chemother. 1971;1988(40):14–25.
Bruck R, Krepel Z, Bar-Meir S. Cimetidine and omeprazole have different effects on hepatic extraction of lidocaine in rats. Gastroenterology. 1990;99:857–9.
Ikeda Y, Oda Y, Nakamura T, Takahashi R, Miyake W, Hase I, Asada A. Pharmacokinetics of lidocaine, bupivacaine, and levobupivacaine in plasma and brain in awake rats. Anesthesiology. 2010;112:1396–403.
Ala-Kokko TI, Löppönen A, Alahuhta S. Two instances of central nervous system toxicity in the same patient following repeated ropivacaine-induced brachial plexus block. Acta Anaesthesiol Scand. 2000;44:623–6.
Müller M, Litz RJ, Hüler M, Albrecht DM. Grand mal convulsion and plasma concentrations after intravascular injection of ropivacaine for axillary brachial plexus blockade. Br J Anaesth. 2001;87:784–7.
Chazalon P, Tourtier JP, Villevielle T, Giraud D, Saïssy JM, Mion G, Benhamou D. Ropivacaine-induced cardiac arrest after peripheral nerve block: successful resuscitation. Anesthesiology. 2003;99:1449–51.
Griffiths JD, Le NV, Grant S, Bjorksten A, Hebbard P, Royse C. Symptomatic local anaesthetic toxicity and plasma ropivacaine concentrations after transversus abdominis plane block for Caesarean section. Br J Anaesth. 2013;110:996–1000.
Ishida T, Sakamoto A, Tanaka H, Ide S, Ishida K, Tanaka S, Mori T, Kawamata M. Transversus abdominis plane block with 0.25 % levobupivacaine: a prospective, randomized, double-blinded clinical study. J Anesth. 2015;29:557–61.
Lacassie HJ, Rolle A, Cortínez LI, Solari S, Corvetto MA, Altermatt FR. Pharmacokinetics of levobupivacaine with epinephrine in transversus abdominis plane block for postoperative analgesia after Caesarean section. Br J Anaesth. 2018;121:469–75.
Copeland S, Ladd L, Gu X, Mather L. The effects of general anesthesia on the central nervous and cardiovascular system toxicity of local anesthetics. Anesth Analg. 2008;106:1429–39.
Englesson S. The influence of acid-base changes on central nervous system toxicity of local anaesthetic agents. I. An experimental study in cats. Acta Anaesthesiol Scand. 1974;18:79–87.
Ohmura S, Kawada M, Ohta T, Yamamoto K, Kobayashi T. Systemic toxicity and resuscitation in bupivacaine-, levobupivacaine-, or ropivacaine-infused rats. Anesth Analg. 2001;93:743–8.
Acknowledgements
We would like to express our appreciation to the staff of the animal experiment facilities of Hiroshima University for their kind assistance. We also express our appreciation to the Maruishi Pharmaceutical Co., Ltd. for the measurement of the blood concentration of levobupivacaine.
Funding
The authors have no sources of funding to declare for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Miyoshi, H., Kato, T., Nakamura, R. et al. Pharmacokinetics of intraperitoneal and subcutaneous levobupivacaine in anesthetized rats. J Anesth 35, 168–174 (2021). https://doi.org/10.1007/s00540-020-02883-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-020-02883-8