Abstract
Purpose
Cases of local anaesthetic systemic toxicity (LAST) periodically occur following transversus abdominal plane (TAP) blocks. The aim of this study was to characterize levobupivacaine absorption pharmacokinetics, with and without epinephrine, and estimate the risk of LAST, based on a previously reported toxic threshold.
Methods
Previously reported data from 11 volunteers receiving ultrasound-guided TAP blocks with and without epinephrine on two independent occasions were analysed. Serial venous concentrations were measured for 90 min. A pharmacokinetic analysis was performed using the NONMEM statistical programme. The use of epinephrine in the solution was included in the analysis of covariates. The associated risk of LAST symptoms associated with different levobupivacaine dose schemes with and without epinephrine was estimated in 1000 simulated subjects.
Results
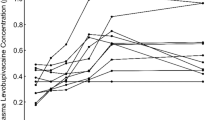
A one-compartment first-order input and elimination model adequately fit the levobupivacaine data. Epinephrine prolonged the levobupivacaine absorption half-life {4.22 [95 % confidence interval (CI) 2.53–6.50] vs. 7.02 [95 % CI 3.74–14.1]; p < 0.05} and reduced its relative bioavailability (0.84; 95 % CI 0.72–0.97; p < 0.05) The derived model predicts that levobupivacaine dose schemes should be halved from 3 mg kg−1 body weight with epinephrine to 1.5 mg kg−1 without epinephrine to obtain a comparable risk of anaesthetic toxicity symptoms of approximately 0.1 %.
Conclusions
Our results strongly support the addition of epinephrine to the local anaesthetic solution, especially when doses of levobupivacaine of >1.5 mg kg−1 are required. Recommendations regarding the maximum allowable doses of local anaesthetics should consider population analysis to determine safer dosage ranges.



Similar content being viewed by others
References
Abdallah FW, Chan VW, Brull R (2012) Transversus abdominis plane block: a systematic review. Reg Anesth Pain Med 37(2):193–209. doi:10.1097/AAP.0b013e3182429531
Griffiths JD, Barron FA, Grant S et al (2010) Plasma ropivacaine concentrations after ultrasound-guided transversus abdominis plane block. Br J Anaesth. doi:10.1093/bja/aeq255
Kato N, Fujiwara Y, Harato M et al (2009) Serum concentration of lidocaine after transversus abdominis plane block. J Anesth 23(2):298–300. doi:10.1007/s00540-008-0721-4
Torup H, Mitchell AU, Breindahl T et al (2012) Potentially toxic concentrations in blood of total ropivacaine after bilateral transversus abdominis plane blocks; a pharmacokinetic study. Eur J Anaesthesiol 29(5):235–238. doi:10.1097/EJA.0b013e328350b0d5
Weiss E, Jolly C, Dumoulin JL et al (2014) Convulsions in 2 patients after bilateral ultrasound-guided transversus abdominis plane blocks for cesarean analgesia. Reg Anesth Pain Med 39(3):248–251. doi:10.1097/AAP.0000000000000088
Naidu RK, Richebe P (2013) Probable Local anesthetic systemic toxicity in a postpartum patient with acute fatty liver of pregnancy after a transversus abdominis plane block. A&A Case Rep 1(5):72–4. doi:10.1097/ACC.0b013e3182973a2f
Corvetto MA, Echevarria GC, De La Fuente N et al (2012) Comparison of plasma concentrations of levobupivacaine with and without epinephrine for transversus abdominis plane block. Reg Anesth Pain Med 37(6):633–637. doi:10.1097/AAP.0b013e31826c330a
Mandema JW, Verotta D, Sheiner LB (1992) Building population pharmacokinetic-pharmacodynamic models. I Models for covariate effects. J Pharmacokinet Biopharm 20:511–528
Bardsley H, Gristwood R, Baker H et al (1998) A comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following intravenous administration to healthy volunteers. Br J Clin Pharmacol 46(3):245–249
Karlsson MO, Sheiner LB (1993) The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm 21(6):735–750
Chalkiadis GA, Abdullah F, Bjorksten AR et al (2013) Absorption characteristics of epidural levobupivacaine with adrenaline and clonidine in children. Paediatr Anaesth 23(1):58–67. doi:10.1111/pan.12074
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276(5309):122–126
Efron B, Tibshirani R (1986) Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci 1(1):54–75
Cortinez LI, Anderson BJ, Holford NH (2015) Dexmedetomidine pharmacokinetics in the obese. Eur J Clin Pharmacol 71(12):1501–1508
Eleveld DJ, Proost JH, Absalom AR et al (2011) Obesity and allometric scaling of pharmacokinetics. Clin Pharmacokinet 50(11):751–753. doi:10.2165/11594080-000000000-00000. Discussion 55–6
Anderson BJ, Holford NH (2008) Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 48:303–332. doi:10.1146/annurev.pharmtox.48.113006.094708
Anderson BJ, Holford NH (2009) Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet 24(1):25–36
Peeters MY, Allegaert K, Blusse van Oud-Alblas HJ et al. Prediction of propofol clearance in children from an allometric model developed in rats, children and adults versus a 0.75 fixed-exponent allometric model. Clin Pharmacokinet 49(4):269–275
Rafi AN (2001) Abdominal field block: a new approach via the lumbar triangle. Anaesthesia 56(10):1024–1026
Lahlou-Casulli M, Chaize - Avril C, Pouliquen E et al (2015) The median effective analgesic dose (ED50) of ropivacaine in ultrasound-guided transversus abdominis plane block for analgesia in reversal of ileostomy: a double-blind up-down dose-finding study. Eur J Anaesthesiol 32(9):640–644
Chalkiadis GA, Eyres RL, Cranswick N et al (2004) Pharmacokinetics of levobupivacaine 0.25% following caudal administration in children under 2 years of age. Br J Anaesth 92(2):218–222
Karmakar MK, Ho AM, Law BK et al (2005) Arterial and venous pharmacokinetics of ropivacaine with and without epinephrine after thoracic paravertebral block. Anesthesiology 103(4):704–711
Krishnan S, Morris RG, Hewett PJ et al (2014) A randomized double-blind clinical trial of a continuous 96-hour levobupivacaine infiltration after open or laparoscopic colorectal surgery for postoperative pain management—including clinically important changes in protein binding. Ther Drug Monit 36(2):202–210. doi:10.1097/FTD.0b013e3182a3772e
McLean AJ, Le Couteur DG (2004) Aging biology and geriatric clinical pharmacology. Pharmacol Rev 56(2):163–184. doi:10.1124/pr.56.2.4
Verbeeck RK (2008) Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol 64(12):1147–1161. doi:10.1007/s00228-008-0553-z
Verbeeck RK, Musuamba FT (2009) Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol 65(8):757–773. doi:10.1007/s00228-009-0678-8
Author contributions
PM wrote and planned the study, analysed the data and wrote the paper; AA wrote and planned the study and analysed the data; MAC wrote and planned the study and performed the research; GCE wrote and planned the study and performed the statistical analyses; LIC: wrote and planned the study, analysed the data and wrote the paper; FRA conceived the study, wrote and planned the study, analysed the data, and wrote the paper. All authors drafted and approved the final manuscript. The content is solely the responsibility of the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
This work was supported by a Research Fund, División de Anestesiología, Escuela de Medicina, Pontificia Universidad Católica de Chile.
Additional information
ClinicalTrials.gov identifier: NCT01596998
Rights and permissions
About this article
Cite this article
Miranda, P., Corvetto, M.A., Altermatt, F.R. et al. Levobupivacaine absorption pharmacokinetics with and without epinephrine during TAP block: analysis of doses based on the associated risk of local anaesthetic toxicity. Eur J Clin Pharmacol 72, 1221–1227 (2016). https://doi.org/10.1007/s00228-016-2086-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2086-1