Abstract
The thiopurine drugs azathioprine and 6-mercaptopurine are widely used for the maintenance of clinical remission in steroid-dependent inflammatory bowel disease (IBD). Thiopurines are recommended to be continued throughout pregnancy in IBD patients, but conclusive safety data in pregnant patients remain still insufficient. On the other hand, a strong association between a genetic variant of nucleoside diphosphate-linked moiety X-type motif 15 (NUDT15 p.Arg139Cys) and thiopurine-induced myelotoxicity has been identified. Pharmacokinetic studies have revealed that thiopurine metabolism is altered in pregnant IBD patients and suggested that the fetus may be exposed to the active-thiopurine metabolite, 6-thioguaninetriphosphate, in the uterus. A recent study using knock-in mice harboring the p.Arg138Cys mutation which corresponds to human p.Arg139Cys showed that oral administration of 6-MP at clinical dose induces a severe toxic effect on the fetus harboring the homozygous or heterozygous risk allele. This suggests that NUDT15 genotyping may be required in both women with IBD who are planning pregnancy (or pregnant) and their partner to avoid adverse outcomes for their infant. The risk to the fetus due to maternal thiopurine use is minimal but there are some concerns that are yet to be clarified. In particular, a pharmacogenomic approach to the fetus is considered necessary.



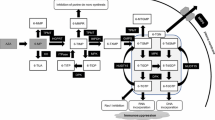
This figure is a modified version from Ref. [27] under Copyright Permission from Elsevier
Similar content being viewed by others
References
Amin J, Huang B, Yoon J, et al. Update 2014: advances to optimize 6-mercaptopurine and azathioprine to reduce toxicity and improve efficacy in the management of IBD. Inflamm Bowel Dis. 2015;21:445–52.
Lee MN, Kang B, Choi SY, et al. Relationship between azathioprine dosage, 6-thioguanine nucleotide levels, and therapeutic response in pediatric patients with IBD treated with azathioprine. Inflamm Bowel Dis. 2015;21:1054–62.
Timmer A, McDonald JW, Tsoulis DJ, et al. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;9: CD000478.
Chande N, Patton PH, Tsoulis DJ, et al. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015. https://doi.org/10.1002/14651858.CD000067.pub3.
Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14:4–22.
van Gennep S, de Boer NK, D’Haens GR, et al. Thiopurine treatment in ulcerative colitis: a critical review of the evidence for current clinical practice. Inflamm Bowel Dis. 2017;24:67–77.
Gearry RB, Barclay ML, Burt MJ, et al. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiol Drug Saf. 2004;13:563–7.
Van Dieren JM, Hansen BE, Kuipers EJ, et al. Meta-analysis: inosine triphosphate pyrophosphatase polymorphisms and thiopurine toxicity in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2007;26:643–52.
Chaparro M, Ordas I, Cabre E, et al. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis. 2013;19:1404–10.
Chang JY, Cheon JH. Thiopurine therapy in patients with inflammatory bowel disease: a focus on metabolism and pharmacogenetics. Dig Dis Sci. 2019;64:2395–403.
Chang JY, Park SJ, Jung ES, et al. Genotype-based treatment with thiopurine reduces incidence of myelosuppression in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020;18:2010-2018.e2.
de Boer NK, de Meij T, van Bodegraven AA. Thiopurines during pregnancy in inflammatory bowel disease: is there a risk for the (unborn) child? Expert Rev Gastroenterol Hepatol. 2013;7:669–71.
McConnell RA, Mahadevan U. Pregnancy and the patient with inflammatory bowel disease: fertility, treatment, delivery, and complications. Gastroenterol Clin North Am. 2016;45:285–301.
Nguyen GC, Seow CH, Maxwell C, et al. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150:734-757.e1.
Chowdhury R, Kane S. Editorial: thiopurine/anti-TNF use during pregnancy-more encouraging safety data. Aliment Pharmacol Ther. 2020;52:1409–10.
Mahadevan U, McConnell RA, Chambers CD. Drug safety and risk of adverse outcomes for pregnant patients with inflammatory bowel disease. Gastroenterology. 2017;152:451-462.e2.
Yang SK, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014;46:1017–20.
Asada A, Nishida A, Shioya M, et al. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2016;51:22–9.
Kakuta Y, Kawai Y, Okamoto D, et al. NUDT15 codon 139 is the best pharmacogenetic marker for predicting thiopurine-induced severe adverse events in Japanese patients with inflammatory bowel disease: a multicenter study. J Gastroenterol. 2018;53:1065–78.
Relling MV, Schwab M, Whirl-Carrillo M, et al. Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin Pharmacol Ther. 2019;105:1095–105.
Moriyama T, Nishii R, Perez-Andreu V, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48:367–73.
Dean L, et al. Azathioprine therapy and TPMT and NUDT15 genotype. In: Pratt VM, Scott SA, Pirmohamed M, et al., editors. Medical genetics summaries. Bethesda: National Center for Biotechnology Information; 2012.
Coenen MJH. NUDT15 genotyping in Caucasian patients can help to optimise thiopurine treatment in patients with inflammatory bowel disease. Transl Gastroenterol Hepatol. 2019;4:81.
Palmieri O, Latiano A, Bossa F, et al. Sequential evaluation of thiopurine methyltransferase, inosine triphosphate pyrophosphatase, and HPRT1 genes polymorphisms to explain thiopurines’ toxicity and efficacy. Aliment Pharmacol Ther. 2007;26:737–45.
Yang JJ, Landier W, Yang W, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015;33:1235–42.
Warner B, Johnston E, Arenas-Hernandez M, et al. A practical guide to thiopurine prescribing and monitoring in IBD. Frontline Gastroenterol. 2018;9:10–5.
Imai T, Kawahara M, Tatsumi G, et al. Thiopurine use during pregnancy has deleterious effects on offspring in Nudt 15(R138C) knock-in mice. Cell Mol Gastroenterol Hepatol. 2021;12:335–7.
Tatsumi G, Kawahara M, Imai T, et al. Thiopurine-mediated impairment of hematopoietic stem and leukemia cells in Nudt 15(R138C) knock-in mice. Leukemia. 2020;34:882–94.
Szymanska E, Kisielewski R, Kierkus J. Reproduction and pregnancy in inflammatory bowel disease—management and treatment based on current guidelines. J Gynecol Obstet Hum Reprod. 2021;50: 101777.
Tavernier N, Fumery M, Peyrin-Biroulet L, et al. Systematic review: fertility in non-surgically treated inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:847–53.
Marri SR, Ahn C, Buchman AL. Voluntary childlessness is increased in women with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:591–9.
Dickson I. Pregnancy safe and beneficial for women with IBD. Nat Rev Gastroenterol Hepatol. 2019;16:454.
Selinger CP, Leong RW, Lal S. Pregnancy related issues in inflammatory bowel disease: evidence base and patients’ perspective. World J Gastroenterol. 2012;18:2600–8.
Cornish J, Tan E, Teare J, et al. A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut. 2007;56:830–7.
Abhyankar A, Ham M, Moss AC. Meta-analysis: the impact of disease activity at conception on disease activity during pregnancy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:460–6.
Broms G, Granath F, Linder M, et al. Birth outcomes in women with inflammatory bowel disease: effects of disease activity and drug exposure. Inflamm Bowel Dis. 2014;20:1091–8.
Reddy D, Murphy SJ, Kane SV, et al. Relapses of inflammatory bowel disease during pregnancy: in-hospital management and birth outcomes. Am J Gastroenterol. 2008;103:1203–9.
van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015;9:107–24.
Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology. 2002;65:240–61.
Sandborn WJ. Azathioprine: state of the art in inflammatory bowel disease. Scand J Gastroenterol Suppl. 1998;225:92–9.
Zimm S, Collins JM, Riccardi R, et al. Variable bioavailability of oral mercaptopurine. Is maintenance chemotherapy in acute lymphoblastic leukemia being optimally delivered? N Engl J Med. 1983;308:1005–9.
Jharap B, de Boer NK, Stokkers P, et al. Intrauterine exposure and pharmacology of conventional thiopurine therapy in pregnant patients with inflammatory bowel disease. Gut. 2014;63:451–7.
Ban L, Tata LJ, Fiaschi L, et al. Limited risks of major congenital anomalies in children of mothers with IBD and effects of medications. Gastroenterology. 2014;146:76–84.
Coelho J, Beaugerie L, Colombel JF, et al. Pregnancy outcome in patients with inflammatory bowel disease treated with thiopurines: cohort from the CESAME Study. Gut. 2011;60:198–203.
Mahadevan U, Long MD, Kane SV, et al. Pregnancy and neonatal outcomes after fetal exposure to biologics and thiopurines among women with inflammatory bowel disease. Gastroenterology. 2021;160:1131–9.
Kanis SL, de Lima-Karagiannis A, de Boer NKH, et al. Use of thiopurines during conception and pregnancy is not associated with adverse pregnancy outcomes or health of infants at one year in a prospective study. Clin Gastroenterol Hepatol. 2017;15:1232-1241.e1.
Cleary BJ, Kallen B. Early pregnancy azathioprine use and pregnancy outcomes. Birth Defects Res A Clin Mol Teratol. 2009;85:647–54.
Hutson JR, Matlow JN, Moretti ME, et al. The fetal safety of thiopurines for the treatment of inflammatory bowel disease in pregnancy. J Obstet Gynaecol. 2013;33:1–8.
Akbari M, Shah S, Velayos FS, et al. Systematic review and meta-analysis on the effects of thiopurines on birth outcomes from female and male patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:15–22.
de Meij TG, Jharap B, Kneepkens CM, et al. Long-term follow-up of children exposed intrauterine to maternal thiopurine therapy during pregnancy in females with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:38–43.
Norgard BM, Nielsen J, Friedman S. In utero exposure to thiopurines/anti-TNF agents and long-term health outcomes during childhood and adolescence in Denmark. Aliment Pharmacol Ther. 2020;52:829–42.
Van Asseldonk DP, de Boer NK, Peters GJ, et al. On therapeutic drug monitoring of thiopurines in inflammatory bowel disease; pharmacology, pharmacogenomics, drug intolerance and clinical relevance. Curr Drug Metab. 2009;10:981–97.
Tiede I, Fritz G, Strand S, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1133–45.
Lennard L. Implementation of TPMT testing. Br J Clin Pharmacol. 2014;77:704–14.
Biemans VBC, Savelkoul E, Gabriels RY, et al. A comparative analysis of tioguanine versus low-dose thiopurines combined with allopurinol in inflammatory bowel disease patients. Aliment Pharmacol Ther. 2020;51:1076–86.
Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56:489–526.
Derijks LJ, Gilissen LP, Engels LG, et al. Pharmacokinetics of 6-thioguanine in patients with inflammatory bowel disease. Ther Drug Monit. 2006;28:45–50.
Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–13.
Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol. 2004;2:731–43.
de Boer NK, Wong DR, Jharap B, et al. Dose-dependent influence of 5-aminosalicylates on thiopurine metabolism. Am J Gastroenterol. 2007;102:2747–53.
Lowry PW, Franklin CL, Weaver AL, et al. Leucopenia resulting from a drug interaction between azathioprine or 6-mercaptopurine and mesalamine, sulphasalazine, or balsalazide. Gut. 2001;49:656–64.
Takahashi K, Bamba S, Morita Y, et al. pH-dependent 5-aminosalicylates releasing preparations do not affect thiopurine metabolism. Digestion. 2019;100:238–46.
Morikubo H, Kobayashi T, Ozaki R, et al. Differential effects of mesalazine formulations on thiopurine metabolism through thiopurine S-methyltransferase inhibition. J Gastroenterol Hepatol. 2021. https://doi.org/10.1111/jgh.15411.
Duley JA, Somogyi AA, Martin JH. The future of thiopurine pharmacogenomics. Pharmacogenomics. 2012;13:1549–52.
Iu YPH, Helander S, Kahlin AZ, et al. One amino acid makes a difference—characterization of a new TPMT allele and the influence of SAM on TPMT stability. Sci Rep. 2017;7: 46428.
Lennard L, Cartwright CS, Wade R, et al. Thiopurine methyltransferase genotype-phenotype discordance and thiopurine active metabolite formation in childhood acute lymphoblastic leukaemia. Br J Clin Pharmacol. 2013;76:125–36.
Nagasaki M, Yasuda J, Katsuoka F, et al. Rare variant discovery by deep whole-genome sequencing of 1,070 Japanese individuals. Nat Commun. 2015;6:8018.
Yamaguchi-Kabata Y, Nariai N, Kawai Y, et al. iJGVD: an integrative Japanese genome variation database based on whole-genome sequencing. Hum Genome Var. 2015;2:15050.
Ban H, Andoh A, Tanaka A, et al. Analysis of thiopurine S-methyltransferase genotypes in Japanese patients with inflammatory bowel disease. Intern Med. 2008;47:1645–8.
Uchiyama K, Nakamura M, Kubota T, et al. Thiopurine S-methyltransferase and inosine triphosphate pyrophosphohydrolase genes in Japanese patients with inflammatory bowel disease in whom adverse drug reactions were induced by azathioprine/6-mercaptopurine treatment. J Gastroenterol. 2009;44:197–203.
Kakuta Y, Naito T, Onodera M, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. 2015;16:280–5.
Zhu X, Wang XD, Chao K, et al. NUDT15 polymorphisms are better than thiopurine S-methyltransferase as predictor of risk for thiopurine-induced leukopenia in Chinese patients with Crohn’s disease. Aliment Pharmacol Ther. 2016;44:967–75.
Wong FC, Leung AW, Kwok JS, et al. NUDT15 variant and thiopurine-induced leukopenia in Hong Kong. Hong Kong Med J. 2016;22:185–7.
Liang DC, Yang CP, Liu HC, et al. NUDT15 gene polymorphism related to mercaptopurine intolerance in Taiwan Chinese children with acute lymphoblastic leukemia. Pharmacogenomics J. 2016;16:536–9.
Xu Y, Qiao YQ, Li HY, et al. NUDT15 genotyping during azathioprine treatment in patients with inflammatory bowel disease: implications for a dose-optimization strategy. Gastroenterol Rep (Oxf). 2020;8:437–44.
Kakuta Y, Izumiyama Y, Okamoto D, et al. High-resolution melt analysis enables simple genotyping of complicated polymorphisms of codon 18 rendering the NUDT15 diplotype. J Gastroenterol. 2020;55:67–77.
Walker GJ, Harrison JW, Heap GA, et al. Association of genetic variants in NUDT15 with thiopurine-induced myelosuppression in patients with inflammatory bowel disease. JAMA. 2019;321:773–85.
Schaeffeler E, Jaeger SU, Klumpp V, et al. Impact of NUDT15 genetics on severe thiopurine-related hematotoxicity in patients with European ancestry. Genet Med. 2019;21:2145–50.
Saarikoski S, Seppala M. Immunosuppression during pregnancy: transmission of azathioprine and its metabolites from the mother to the fetus. Am J Obstet Gynecol. 1973;115:1100–6.
Hutson JR, Lubetsky A, Walfisch A, et al. The transfer of 6-mercaptopurine in the dually perfused human placenta. Reprod Toxicol. 2011;32:349–53.
Maruyama H, Tada K, Fujiwara T, et al. Utility of maternal 6-thioguanine nucleotide levels in predicting neonatal pancytopenia. AJP Rep. 2013;3:25–8.
Thomas C, Monteil-Ganiere C, Mirallie S, et al. A severe neonatal lymphopenia associated with administration of azathioprine to the mother in a context of Crohn’s disease. J Crohns Colitis. 2018;12:258–61.
Flanagan E, Wright EK, Hardikar W, et al. Maternal thiopurine metabolism during pregnancy in inflammatory bowel disease and clearance of thiopurine metabolites and outcomes in exposed neonates. Aliment Pharmacol Ther. 2021;53:810–20.
Watters JW, Zhang W, Meucci MA, et al. Analysis of variation in mouse TPMT genotype, expression and activity. Pharmacogenetics. 2004;14:247–54.
Nishii R, Moriyama T, Janke LJ, et al. Preclinical evaluation of NUDT15-guided thiopurine therapy and its effects on toxicity and antileukemic efficacy. Blood. 2018;131:2466–74.
Moriyama T, Yang YL, Nishii R, et al. Novel variants in NUDT15 and thiopurine intolerance in children with acute lymphoblastic leukemia from diverse ancestry. Blood. 2017;130:1209–12.
Acknowledgements
This work was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP20gm1010008h9904 (AA) and 19ek0410056h0001 (YK), and in part by a Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan under Grant Number 19K08811(MK), 21K07955(YK) and 18K08002(AA), and in part by Health and Labor Sciences Research Grants for Research on Intractable Diseases from the Ministry of Health, Labor and Welfare of Japan under Grant Number 20FC1037 (AA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AA receiving lecture fee from Janssen, Takeda, AbbVie, Tanabe-Mitsubishi. All other authors declare that they have no conflict of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andoh, A., Kawahara, M., Imai, T. et al. Thiopurine pharmacogenomics and pregnancy in inflammatory bowel disease. J Gastroenterol 56, 881–890 (2021). https://doi.org/10.1007/s00535-021-01805-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-021-01805-z