Abstract
Background
Proton pump inhibitor (PPI)-refractory gastroesophageal reflux disease (GERD) leads to a clinical decline in the quality of life (QOL). Therefore, new treatment options are needed. We performed a multicenter, randomized, parallel-group exploratory trial to determine the efficacy of hangeshashinto (HST) in patients with PPI-refractory GERD.
Methods
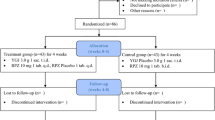
We enrolled 78 patients with PPI-refractory GERD for standard PPI regimens for at least 4 weeks and randomly assigned patients to receive either a combination of usual dose of rabeprazole (10 mg/day) + HST (7.5 g/day; HST group) or a double dose of rabeprazole (20 mg/day; double-dose PPI group). The primary end points were the extent of improvement in FSSG (Frequency Scale for the Symptoms of GERD) score and the change over time in FSSG score.
Results
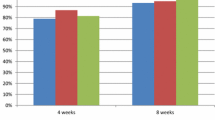
There was no significant difference in terms of the improvement degree of the FSSG score between the two groups. Although the total FSSG score and reflux syndrome score decreased significantly for both groups over time (p < 0.001), the acid-related dyspepsia (ARD) score decreased significantly in the HST group from 1 week after drug administration (p < 0.05), indicating an improvement in the condition earlier than in the double-dose PPI group. Moreover, in examinations concerning BMI and age, the HST group had a significantly higher improvement degree of ARD score in patients with BMI < 22 (p < 0.05) and aged < 65 years (p < 0.05) than the double-dose PPI group.
Conclusions
HST may be beneficial for patients with PPI-refractory GERD, particularly in non-obese and non-elderly patients with dyspepsia symptoms.



Similar content being viewed by others
References
Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20.
Iwakiri K, Kinoshita Y, Habu Y, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol. 2016;51:751–67.
Fujiwara Y, Arakawa T. Epidemiology and clinical characteristics of GERD in the Japanese population. J Gastroenterol. 2009;44:518–34.
Fass R, Shapiro M, Dekel R, et al. Systematic review: proton-pump inhibitor failure in gastro-oesophageal reflux disease—where next? Aliment Pharmacol Ther. 2005;22:79–94.
Iwakiri K, Kawami N, Sano H, et al. Acid and non-acid reflux in Japanese patients with non-erosive reflux disease with persistent reflux symptoms, despite taking a double-dose of proton pump inhibitor: a study using combined pH-impedance monitoring. J Gastroenterol. 2009;44:708–12.
G-PRIDE Study Group, Tominaga K, Kato M, et al. A randomized, placebo-controlled, double-blind clinical trial of rikkunshito for patients with non-erosive reflux disease refractory to proton-pump inhibitor: the G-PRIDE study. J Gastroenterol. 2014;49:1392–405.
Sakata Y, Tominaga K, Kato M, et al. Clinical characteristics of elderly patients with proton pump inhibitor-refractory non-erosive reflux disease from the G-PRIDE study who responded to rikkunshito. BMC Gastroenterol. 2014;14:116.
Harasawa S, Miwa T. The efficacy of Hangeshashinto on gastric motility disorders. Prog Med. 1993;13:2533–9 (Japanese).
Gochi A, Hirose S, Sato K, et al. The effect of Hange-shashin-to and Rikkunshi-to against the digestive symptoms after gastrectomy. Jpn J Gastroenterol Surg. 1995;28:961–5 (Japanese).
Aoyama T, Nishikawa K, Takiguchi N, et al. Double-blind, placebo-controlled, randomized phase II study of TJ-14 (hangeshashinto) for gastric cancer chemotherapy-induced oral mucositis. Cancer Chemother Pharmacol. 2014;73:1047–54.
Matsuda C, Munemoto Y, Mishima H, et al. Double-blind, placebo-controlled, randomized phase II study of TJ-14 (Hangeshashinto) for infusional fluorinated-pyrimidine-based colorectal cancer chemotherapy-induced oral mucositis. Cancer Chemother Pharmacol. 2015;76:97–103.
Yamaguchi O, Kawashima A, Shiono A, et al. Hange-Shashin-to for preventing diarrhea during afatinib therapy. Gan To Kagaku Ryoho. 2015;42:581–3 (Japanese).
Hoshi N, Kofunato Y, Yashima R, et al. Treating side effects of FOLFIRINOX–a study of the effect of Hange-shashin-to on preventing diarrhea. Gan To Kagaku Ryoho. 2015;42:2364–6 (Japanese).
Fukamachi H, Matsumoto C, Omiya Y, et al. Effects of Hangeshashinto on growth of oral microorganisms. Evid Based Complement Altern Med. 2015;2015:1–6.
Kase Y, Hayakawa T, Ishige A, et al. The Effects of Hange-shashin-to on the content of prostaglandin E2 and water absorption in the large intestine of rats. Biol Pharm Bull. 1997;20:954–7.
Takeuchi T, Kawaguchi S, Takahashi Y, et al. Su1132 efficacy of Hange-shashinto on gastrointestinal symptoms in patients with gastroesophageal reflux disease refractory to proton pump inhibitor therapy. Gastroenterology. 2016;150:S478.
Kusano M, Shimoyama Y, Sugimoto S, et al. Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol. 2004;39:888–91.
Kusano M, Shimoyama Y, Kawamura O, et al. Proton pump inhibitors improve acid-related dyspepsia in gastroesophageal reflux disease patients. Dig Dis Sci. 2007;52:1673–7.
Svedlund J, Sjödin I, Dotevall G. GSRS—a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–34.
Tominaga K, Iwakiri R, Fujimoto K, et al. Rikkunshito improves symptoms in PPI-refractory GERD patients: a prospective, randomized, multicenter trial in Japan. J Gastroenterol. 2012;47:284–92.
Domingues G, Moraes-Filho JPP, Fass R. Refractory heartburn: a challenging problem in clinical practice. Dig Dis Sci. 2018;63:577–82.
Altomare A, Guarino MP, Cocca S, et al. Gastroesophageal reflux disease: update on inflammation and symptom perception. World J Gastroenterol. 2013;19:6523–8.
Kondo T, Oshima T, Tomita T, et al. Prostaglandin E 2 mediates acid-induced heartburn in healthy volunteers. Am J Physiol-Gastrointest Liver Physiol. 2013;304:G568–73.
Kondo T, Oshima T, Tomita T, et al. The nonsteroidal anti-inflammatory drug diclofenac reduces acid-induced heartburn symptoms in healthy volunteers. Clin Gastroenterol Hepatol. 2015;13(1249–1255):e1.
Kondo T, Sei H, Yamasaki T, et al. A novel prostanoid EP1 receptor antagonist, ONO-8539, reduces acid-induced heartburn symptoms in healthy male volunteers: a randomized clinical trial. J Gastroenterol. 2017;52:1081–9.
Kono T, Kaneko A, Matsumoto C, et al. Multitargeted effects of hangeshashinto for treatment of chemotherapy-induced oral mucositis on inducible prostaglandin e2 production in human oral keratinocytes. Integr Cancer Ther. 2014;13:435–45.
Takasuna K, Hagiwara T, Watanabe K, et al. Optimal antidiarrhea treatment for antitumor agent irinotecan hydrochloride (CPT-11)-induced delayed diarrhea. Cancer Chemother Pharmacol. 2006;58:494–503.
Miyashita T, Kono T, Matsui D, et al. Preventive effect of oral hangeshashinto (TJ-14) on the development of reflux-induced esophageal cancer. Surgery. 2018;164:49–55.
Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors. JAMA Intern Med. 2016;176:172.
Mössner J. The indications, applications, and risks of proton pump inhibitors. Dtsch Arztebl Int. 2016;113:477.
Dunbar KB, Agoston AT, Odze RD, et al. Association of acute gastroesophageal reflux disease with esophageal histologic changes. JAMA. 2016;315:2104–12.
Ren LH, Chen WX, Qian LJ, et al. Addition of prokinetics to PPI therapy in gastroesophageal reflux disease: a meta-analysis. World J Gastroenterol. 2014;20:2412–9.
Miwa H, Inoue K, Ashida K, et al. Randomised clinical trial: efficacy of the addition of a prokinetic, mosapride citrate, to omeprazole in the treatment of patients with non-erosive reflux disease—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:323–32.
Takeuchi T, Takahashi Y, Kawaguchi S, et al. Therapy of gastroesophageal reflux disease and functional dyspepsia overlaps with symptoms after usual-dose proton pump inhibitor: acotiamide plus usual-dose proton pump inhibitor versus double-dose proton pump inhibitor: Therapy of GERD and FD overlaps. J Gastroenterol Hepatol. 2018;33:623–30.
Shah SL, Lacy BE, DiBaise JK, et al. The impact of obesity on oesophageal acid exposure time on and off proton pump inhibitor therapy. Aliment Pharmacol Ther. 2015;42:1093–100.
Utumi Y, Iseki E, Murayama N, et al. Effect of Rikkunshi-to on appetite loss found in elderly dementia patients: a preliminary study: rikkunshi-to and elderly dementia patients. Psychogeriatrics. 2011;11:34–9.
Pilotto A, Franceschi M, Paris F. Recent advances in the treatment of GERD in the elderly: focus on proton pump inhibitors: treatment of GERD in the elderly. Int J Clin Pract. 2005;59:1204–9.
Mantani N, Oka H, Watanabe T, et al. Incidence of liver injury related to kampo medicine containing Scutellaria baicalensis at our clinic. Kampo Med. 2017;68:377–81 (Japanese).
Acknowledgements
Kazuhide Higuchi and Toshihisa Takeuchi contributed to the concept and design of the study. We are grateful to the GERD-4 Study Group (Tetsuo Arakawa, Kazuma Fujimoto, Motoyasu Kusano, Kazunari Tominaga) for providing a useful reference for the design of this study. All authors approved the final version of the manuscript. We thank Nouvelle Place Inc. Tokyo, Japan, for their great support on data management and statistical analysis. We would like to thank Editage (www.editage.jp) for English language editing. We thank Chinatsu Saito, Yoshinori Ozaki of Tsumura Co., Ltd. Tokyo, Japan, who received information on hangeshashinto for research planning. This research was funded by Tsumura Co., Ltd. Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kazuhide Higuchi has received honoraria from Tsumura Co,. Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takeuchi, T., Hongo, H., Kimura, T. et al. Efficacy and safety of hangeshashinto for treatment of GERD refractory to proton pump inhibitors. J Gastroenterol 54, 972–983 (2019). https://doi.org/10.1007/s00535-019-01588-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-019-01588-4