Abstract
Background
In this study, survival and cause of death were investigated in patients with Crohn’s disease (CD) at a tertiary referral center.
Methods
A database was created based on the medical records of 1108 CD patients who had a history of visiting our hospital to investigate background characteristics, cumulative survival rates from diagnosis, causes of death, and the standardized mortality ratio (SMR) for each cause of death. A follow-up questionnaire survey of patients followed up inadequately was also conducted. The cumulative survival rate from diagnosis was determined using the life table method and compared with that of a sex- and age-matched population model from the year 2000.
Results
The study included 1108 patients whose mean age at diagnosis was 25.6 ± 10.8 years. The mean duration of follow-up was 14.6 ± 9.4 years, and there were 52 deaths. The cumulative survival rate was significantly lower 25 years after the diagnosis of CD (91.7%) than in the standard population model (95.7%). SMRs for both all causes [3.5; 95% confidence interval (CI): 2.7–4.6] and CD-specific causes (36.7; 95% CI 26.1–51.6) were high. Among the CD-specific causes, SMRs were especially high for small intestine and colorectal cancers, gastrointestinal diseases including intestinal failure (IF), perioperative complications, and amyloidosis.
Conclusion
The SMRs for both all causes and CD-specific causes were high in CD patients. CD-specific causes including intestinal cancer, IF, perioperative complications, and amyloidosis showed especially high SMRs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) of unknown etiology. It often develops at an early age and can affect the entire gastrointestinal tract from the oral cavity to the anus. It is also known to frequently require intestinal resection due to the development of intestinal complications such as stenosis and fistula through repeated relapses or recurrences [1,2,3,4]. Although there are some reports of the long-term course of patients with this disease, there are very few reports related to their survival and cause of death; moreover, most previous reports are derived from population-based cohort studies, and there are very few, long-term, hospital-based, cohort studies with a long duration of follow-up and a large sample size [5,6,7,8,9,10,11,12,13,14]. In the present study, a database based on the medical records of CD patients at our hospital with the addition of information obtained from a questionnaire survey was created, and the long-term prognosis of CD patients was investigated.
Methods
Study design and populations
This was a single-center, retrospective, cohort study conducted at Fukuoka University Chikushi Hospital. Among the patients with a definitive diagnosis of CD who had a history of visiting our hospital between May 1967 and December 2015, patients with a history of treatment for at least 6 months since diagnosis were selected, and a database based on their medical records was subsequently created [3]. Patients who were followed up for less than 6 months after diagnosis were excluded. First, sex, age at diagnosis, date of diagnosis, smoking history, disease type at diagnosis, presence/absence of perianal disease, survival to the last observed day, surgical history, and use of medications (aminosalicylates, corticosteroids, thiopurines, and anti-tumor necrosis factor [TNF] agents) were determined from the medical records. Subsequently, a questionnaire survey of the patients who had not visited our hospital for at least 3 years was conducted between May 2015 and May 2016.
In the present study, (1) the cumulative mortality of CD patients was compared with that of the standard population, (2) the causes of death in CD and the standardized mortality ratios (SMRs) by cause of death were investigated, and (3) background characteristics that affect mortality in CD patients were investigated.
Duration of follow-up
Duration of follow-up was defined as from the date of diagnosis to the last observed day. The date of diagnosis was determined based on medical records at our hospital, and the last observed day was the date of the final visit to our hospital according to the medical records, or the date of survey response or date of death for patients whose information could be collected through the follow-up questionnaire survey.
Ever-use of medicine
The use of medications was reviewed for the duration of follow-up in all patients, and the use of aminosalicylates, corticosteroids, thiopurines, and anti-TNF agents was examined. Duration of medication use, dose, and side effects were not taken into consideration.
Diagnosis and treatment of CD
CD was diagnosed based on clinical symptoms, endoscopic findings, X-ray findings, and histological findings in accordance with the diagnostic criteria for CD proposed by the Japanese Ministry of Health, Labour and Welfare [15,16,17]. Furthermore, using the Montreal classification, disease location at diagnosis was classified into ileal type, colonic type, or ileocolonic type, and disease behavior at diagnosis was classified into inflammatory, stricturing, or penetrating type [18].
Assessment of mortality
Date and cause of death were determined based on medical records, and for the patients whose deaths were identified through the follow-up questionnaire survey were determined through an inquiry to the facility where they passed away. As in previous reports, the causes of death were classified in accordance with the International Classification of Diseases 10th revision (ICD-10) [13, 14, 19].
Gastrointestinal disease (excluding non-alcoholic liver disease, ICD codes K00–K70, 77–93) included intestinal complications of CD such as intestinal obstruction and perforation, perioperative complications, state of undernutrition due to short-bowel syndrome, and sudden death of unknown cause that occurred during home parenteral nutrition (HPN) or the active stage of the disease. Non-alcoholic liver disease (ICD code: K71–76) included chronic and progressive liver diseases that were not considered associated with CD.
There are no previous reports of the SMRs for deaths related to CD. Therefore, with the aim to calculate CD-specific mortality, the total mortality for small intestinal cancer (ICD code: C17), colorectal cancer (CRC) (ICD code: C18–21), and amyloidosis (ICD code: E85), as well as gastrointestinal diseases, as CD-specific causes was determined, and the numbers of expected cases of death and SMRs were calculated from the above mortality and person-years in the sex- and age-matched population model.
Statistical analysis
-
1.
Expected and observed cumulative survival rates from diagnosis
The age by sex-specific death rates of the Japanese population in 2000 were applied to the distribution of the age groups of the study population by sex to estimate the yearly expected survival rate of the general population during the follow-up period. The death rates for 5-year age bands of both sexes were used in this study. According to the past reports, the age by sex-specific mortality of the Japanese population in 2000 [20], which was almost the median of the year of diagnosis, was used [13]. The life table method was used to estimate the cumulative survival rate and 95% confidence intervals of the study subjects.
-
2.
SMRs of patients with CD
The SMR is the ratio of the observed number of deaths among the patients with CD in this cohort study to the expected number of deaths in the study population under the assumption that the age by sex-specific mortality rates for the study population are the same as those for a reference population. The expected number of deaths was calculated by multiplying the person-years of the study population by the age by sex-specific death rates of the Japanese population in 2000. The SMRs were calculated for 5-year age bands of both sexes in this study.
-
3.
Factors associated with mortality of patients with CD
Hazard ratios of death and their 95% confidence intervals were estimated with a Cox proportional hazards model. Age was treated as a continuous variable, while other factors were treated as indicator variables.
-
4.
Number of intestinal resections and mortality in patients with CD.
Number of intestinal resection was classified into three categories: 0, 1–4, 5 or more. A Chi-squared test was used to evaluate if the number of intestinal resections was related to the death rate. The dose-dependent trend was tested by evaluating the regression coefficient when the categories were treated as equally spaced numerical variables in Cox’s model
Person-years at risk were computed from the date of CD diagnosis until the date of death, loss to follow-up, or end of follow-up.
All statistical analyses were conducted using the Statistical Analysis System (SAS Institute, Cary, NC, USA) package. A two-sided P less than 0.05 was considered significant.
Ethical considerations
This study was conducted in accordance with the 1964 Helsinki Declaration and its subsequent amendments. Personal information listed in the medical records or obtained through questionnaire surveys, including informed consent, was managed on a database such that individuals were anonymized. This study was approved by the institutional review board of Fukuoka University Chikushi Hospital (R14-011, 2014.6.4–2017.2.28).
Results
Patient populations and background characteristics
This study included 1108 patients whose duration of follow-up was at least 6 months from among the 1165 CD patients who were diagnosed after May 1967 and had a history of visiting our hospital to December 2015. A questionnaire survey was distributed to 303 CD patients who did not have a history of visiting our hospital for at least 3 years, and information on their present condition (alive, dead), cause of death, last observed day, smoking history, surgical history, and previously used medications was added from the 106 responses. It was, therefore, possible to obtain information on background characteristics and survival to the last observed day for all patients. The patients’ clinical characteristics are shown in Table 1. There were 758 men and 350 women who were 7–76 years old at the time of diagnosis. The mean age at diagnosis was 25.6 ± 10.8 years, and the mean duration of follow-up was 14.6 ± 9.4 years (range 0.5–47.2 years). The study included 16,199 person-years, and 583 patients (52.6%) were diagnosed before 2000. Disease location at diagnosis was ileal type in 386 patients (34.8%), colonic type in 197 patients (17.8%), and ileocolonic type in 525 patients (47.4%). Disease behavior was inflammatory in 531 patients (47.9%), stricturing in 365 patients (32.9%), penetrating in 208 patients (18.8%), and unknown in 4 patients (0.4%). A perianal fistula was present at diagnosis in 594 patients (53.6%). There were 303 current smokers (27.3%), 610 never-smokers (55.1%), and 117 ex-smokers (10.6%).
There were 662 patients (59.7%) who had undergone intestinal surgery by the last observed day. The mean number of surgeries per patient was 1.2 ± 1.4. Previously used medications included aminosalicylates in 864 patients (77.9%), corticosteroids in 346 patients (31.2%), thiopurines in 423 patients (38.1%), and anti-TNF agents in 570 patients (51.4%).
Overall mortality
Fifty-two deaths occurred among the 1108 patients with CD. Among the 52 deaths, 9 were reported through the additional questionnaire survey. The background characteristics of the deceased patients are shown in Table 2. Of the 52 patients who died, 32 were men (61.5%). The mean age at diagnosis was 29.1 ± 14.0 years, the mean age at death was 48.1 ± 14.6 years, and the mean duration of follow-up from diagnosis to death was 18.3 ± 8.8 years. Disease location at diagnosis was ileal type in 15 patients (28.9%), colonic type in 6 patients (11.5%), and ileocolonic type in 31 patients (59.6%). Disease behavior at diagnosis was inflammatory in 21 patients (40.4%), stricturing in 19 patients (36.5%), and penetrating in 12 patients (23.1%). There were 31 patients (59.6%) presenting with perianal disease, 2 current smokers (3.9%), and 45 patients (86.5%) who had undergone surgery during the follow-up period. The mean number of surgeries per patient was 2.3 ± 1.8. Previously used medications were aminosalicylates in 29 patients (55.8%), corticosteroids in 26 patients (50.0%), thiopurines in 12 patients (23.1%), and anti-TNF agents in 21 patients (40.4%).
Expected and observed cumulative survival rates from diagnosis
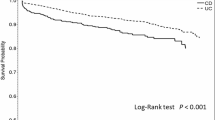
The cumulative survival rates from diagnosis in CD patients are shown in Fig. 1. The cumulative survival rates among CD patients were 99.3% (95% CI 98.8–99.9) after 10 years (expected cumulative survival rate: 98.3%, 95% CI 97.5–99.0%), 95.1% (95% CI 93.3–96.9%) after 20 years (expected cumulative survival rate: 96.5%, 95% CI 95.5–97.6%), and 87.1% (95% CI 82.8–91.4%) after 30 years (expected cumulative survival rate: 94.9%, 95% CI 93.6–96.1%). The cumulative survival rate of CD patients in the first 24 years since diagnosis was not different from that of the standard population model. However, it was significantly lower in CD patients (91.7%; 95% CI 89.0–94.4%) at 25 years after diagnosis compared to that in the standard population model (95.7%; 95% CI 94.5–96.9%), and this pattern continued thereafter.
Causes of death in CD and the SMRs by cause of death
The numbers of deaths and SMRs by cause of death in CD patients are shown in Table 3. Fifty-two of the 1108 CD patients died. The causes of death were: malignant tumor (n = 19; small intestinal cancer, n = 2; CRC, n = 11; lung cancer, n = 2; lymphoma, n = 2; brain tumor, n = 1; and cancer of unknown primary, n = 1), gastrointestinal disease (n = 15), liver disease (n = 3), respiratory disease (n = 2), cardiovascular disease (n = 3), suicide (n = 3), metabolic disease (n = 5), and traffic injuries (n = 1). Information about the cause of death was not provided on the questionnaire survey in one case. There were a total of 33 deaths related to CD, including small intestinal cancer, CRC, gastrointestinal disease, and amyloidosis (pathologically secondary amyloidosis as shown by immunostaining AA amyloid). Gastrointestinal disease (n = 15) consisted of intestinal failure (IF) (n = 7), severe (fistulous) disease including perioperative complications (n = 6), and intestinal perforation (n = 2). Amyloidosis (n = 5) consisted of kidney failure (n = 3) and massive bleeding (n = 2).
SMRs
Fifty-two all-cause deaths were observed in this study, while the expected value calculated from the mortality in the sex- and age-matched general population was 14.8. Thus, the SMR for all-cause mortality was 3.5 (95% CI 2.7–4.6).
Analysis by disease revealed a high SMR of 5.4 (95% CI 3.5–8.5) for malignant tumors. SMRs were especially high for small intestinal cancer at 200 (95% CI 500.1–7997.1), CRC at 29.7 (95% CI 16.5–53.7), and brain tumor at 12.5 (95% CI 1.8–88.7). In addition, SMRs were also high for gastrointestinal disease at 48.4 (95% CI 29.2–80.3), non-alcoholic liver disease at 14.3 (95% CI 4.6–44.3), and amyloidosis at 1000 (95% CI 416.2–2402.6). However, high SMRs were not observed for many conditions, such as respiratory disease (1.4; 95% CI 0.4–5.7), cardiovascular disease (1.3; 95% CI 0.4–3.9), suicide (1.2; 95% CI 0.4–3.6), and traffic injuries (0.7; 95% CI 500.1–7997.1). The SMR for CD-specific causes of death, which included small intestinal cancer, CRC, gastrointestinal disease, and metabolic disease, was high at 36.7 (95% CI 26.1–51.6).
Thus, the results showed that many deaths of CD patients were caused by intestinal cancer, IF, severe (fistulous) disease including perioperative complications, and amyloidosis.
Factors associated with mortality in patients with CD
Factors associated with mortality are shown in Table 4. Sex, calendar year, disease location and behavior at diagnosis, smoking habits, perianal fistula, thiopurines, and use of anti-TNF agents were not significant factors. However, old age at diagnosis (≥ 60 years) [hazard ratio (HR) 19.93; 95% CI 5.56–71.54] and use of corticosteroids (HR 1.94; 95% CI 1.12–3.25) had high HRs.
Number of intestinal resections and the death rate
The number of intestinal resections and the death rate in all subjects and in patients followed up more than 25 years were examined (Table 5). A higher number of surgeries was significantly associated with higher mortality during the entire follow-up period (Table 5, p < 0.01 by Chi-squared test, p for trend < 0.01), as well as in patients who were followed up for 25 years and more (Table 5, p = 0.03 by Chi-squared test, p for trend = 0.02).
Discussion
The results of long-term follow-up of CD patients in the present study showed that the cumulative survival rate decreased and SMR increased ≥ 25 years after diagnosis. Among individual complications, CD-specific causes, such as intestinal cancer, IF, severe (fistulous) diseases including perioperative complications, and amyloidosis, developed ≥ 20 years after diagnosis, increasing mortality.
There are several reports primarily from Western countries concerning the survival and causes of death of CD patients. It has recently been reported that mortality is similar or slightly higher in CD patients than in the general population [5,6,7,8,9,10,11,12,13,14]. However, most data are derived from population-based studies, which are considered not conducive to patient follow-up or detailed investigation of the causes of deaths, and there are very few, long-term, hospital-based studies with a large sample size [5, 6, 14, 21,22,23,24,25]. Therefore, this hospital-based cohort study was conducted at a tertiary referral center in Japan to investigate survival, cause of death, and factors that affect mortality of CD patients in detail.
The cumulative survival rates of CD patients from diagnosis were 98.9% at 10 years, 94.0% at 20 years, and 86.7% at 30 years according to Lee et al. from Korea [14], and the rates were generally consistent with the results of the present study. Some reports compared the cumulative survival rate from diagnosis with that of the standard population model and found no significant differences between the two groups [5, 8, 11]. Consistent with previous reports, the present study results also did not show significant differences for 25 years from diagnosis. However, longer follow-up demonstrated that the cumulative survival rate decreased significantly ≥ 25 years from diagnosis. These findings suggest that long-term follow-up of 20 years or longer may show a decrease in cumulative survival among CD patients.
Table 6 shows SMRs for all causes from previous hospital-based studies [5, 6, 14, 21,22,23,24,25]. Prior et al. [21] and Weterman et al. [22] reported high SMRs, but recent reports did not find increased SMRs. Except for the study by Lee et al., previous reports encompassed a low number of person-years (range 2712–7424), which takes into account the number of patients and the duration of follow-up. Furthermore, compared to the report by Lee at al., the present study had a longer mean duration of follow-up (14.6 years) with 16,197 person-years. It is evident from Fig. 1 that the cumulative survival rate > 25 years after diagnosis was decreased, indicating that the present study might be informative, since there are no other studies with both high person-years at risk and a long duration of follow-up.
Regarding SMRs by disease, previous reports showed that SMRs were high for neoplasms, especially small intestinal cancer and CRC, as well as gastrointestinal diseases [13, 14, 26, 27]. As in previous studies, the present study also found high SMRs for malignant tumors, especially small intestinal cancer and CRC, as well as gastrointestinal disease and amyloidosis, and not very high SMRs for respiratory and cardiovascular disease. The SMR of all causes was high in the present study, likely because of the high SMR for CD-specific causes.
In the present study, the SMR for gastrointestinal disease was high, because the data showed that the number of intestinal resections was significantly related to the death rate (Table 5). CD induces intestinal complications over the long course of the disease, requiring repeated surgeries. Frequent surgeries and intestinal complications can result in short-bowel syndrome and IF. According to Watanabe et al., IF was observed relatively frequently at a specialized hospital, with an incidence of 3.6% at 10 years and 8.5% at 20 years after initial surgery for CD, and the mortality after diagnosis of IF was 3.7% at 5 years and 8.9% at 10 years [28]. HPN-related mortality according to reports from overseas ranges from 2 to 28% at 5 years, which is by no means low [29]. Loly et al. collected data from numerous cases and stated that the mortality of CD patients who received HPN was 10% at 5 years, and Limketkai et al. stated that mortality caused by IF was 6% at 1 year and up to 20% at 4 years [30, 31]. It could be expected that mortality due to intestinal complications, as well as short-bowel syndrome and IF caused by such complications, would be high, and the SMR for gastrointestinal disease is considered to be high, similar to previous reports.
Malignant tumors had the second highest SMR in the present study. Recently, Caini et al. from Italy conducted long-term follow-up of 231 CD patients and compared SMRs by causes of death. Their results showed that the SMR was 1.79 for all causes (95% CI 1.39–2.27), and it was very high for cancer, at 2.57 (95% CI 1.28–5.13) [32]. Furthermore, most reports have demonstrated that the SMR is higher for lower gastrointestinal cancer among malignant tumors. Reports from Western countries have shown the standardized incidence ratio (SIR) to be 2.5 (95% CI 1.3–4.7) for CRC and 33.2 (95% CI 15.9–60.9) for small-bowel cancer [33]. It has also been reported in Japan that the SIR for CRC is 2.79–5.8 [34, 35]. Higashi et al. reported that the frequency of comorbid CRC in CD from multiple facilities specialized for IBD was 3.5% (122/3454 patients), and that the 5-year survival rate of CRC was 88% in Stage I, 68% in Stage II, 71% in Stage IIIa, 25% in Stage IIIb, and 0% in Stage IV [36]. Of these cases, 91% of the diagnosed gastrointestinal cancers were advanced cancers. In addition, they claimed that comorbid CRC has increased markedly in Japan since 2008. Sugita et al. analyzed 20 CD patients with rectal and anal cancers, and they reported a mortality of 33% after 36 months [37]. As described here, the incidences of intestinal cancer and anal cancer have also increased in Japan, and mortality associated with these diseases is not low.
One of the major differences between this study and previous reports is that the SMR for amyloidosis was very high. The prevalence of comorbid secondary amyloidosis in patients with CD has been reported to be 2.5–5.3% in Japan and 0.9–5.6% in Western countries [38,39,40,41,42]. It is considered that, when amyloidosis develops in conjunction with CD, serious complications including kidney failure ensue, ultimately increasing mortality [42]. A recent study from the USA showed that mortality increases in the presence of secondary amyloidosis in hospitalized CD patients [41]. A report by Miyaoka from Japan stated that secondary amyloidosis does not occur frequently in CD (2.5%), but that 40% of these patients died, indicating that the prognosis is poor with secondary amyloidosis [40]. In addition, a long-term outcome report by Weterman et al. showed that 4 of 64 deaths (6.3%) were due to amyloidosis [22], indicating that this is not rare. Patients are diagnosed with amyloidosis between 12.0 and 25.0 years after the diagnosis of CD [38, 40, 43], and they die of complications such as kidney failure. Thus, death caused by amyloidosis may only be captured by performing long-term follow-up, as demonstrated in the present study.
The limitations of this study are as follows. It is very difficult to obtain long-term outcomes including mortality rates in a cohort group, because CD patients have younger disease onset, and follow-up for more than 20 years is extremely difficult due to their higher social activity and frequent changes in residence. Because we have an affiliated hospital network and added a questionnaire survey, we succeeded in gathering sufficient data related to mortality and cause of death over a long term.
Because the present study was a retrospective cohort study, disease location and behavior observed at initial examination were not significant factors associated with mortality. Furthermore, while the usefulness of anti-TNF-α agents and a decrease in the intestinal resections performed for refractory CD have been suggested recently, there was no association with the use of medications [44,45,46,47]. Nutritional therapy was not considered in the present study; it was considered difficult to elucidate the contribution of nutritional therapy to mortality since the data were old and retrospective, and the dose and duration of the therapy were not known in many of the patients. Further investigations will be necessary to clarify the associations with treatment and changes in disease type.
Conclusion
The long-term follow-up of CD patients in the present study showed that the cumulative survival rate decreases and SMR increases ≥ 25 years after diagnosis. Among individual complications, CD-specific causes such as intestinal cancer, IF, severe (fistulous) diseases including perioperative complications, and amyloidosis developed ≥ 20 years after diagnosis, increasing the mortality. In the future, it may be possible to improve the prognosis by predicting these serious complications or by diagnosing them at an early stage.
Change history
10 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00535-021-01830-y
Abbreviations
- CD:
-
Crohn’s disease
- CI:
-
Confidence interval
- CRC:
-
Colorectal cancer
- HPN:
-
Home parenteral nutrition
- HR:
-
Hazard ratio
- IBD:
-
Inflammatory bowel disease
- ICD:
-
International Classification of Diseases
- IF:
-
Intestinal failure
- SMR:
-
Standardized mortality ratio
- SIR:
-
Standardized incidence ratio
- TNF:
-
Tumor necrosis factor
References
Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, et al. The nature history of adult Crohn’s disease in population based cohorts. Am J Gastroenterol. 2010;105:289–97.
Lakatos PL, Golovics PA, David G, et al. Has there been a change in the natural history of Crohn’s disease? Surgical rates and medical management in a population-based inception cohort from Western Hungary between 1977–2009. Am J Gastroenterol. 2012;107:579–88.
Sato Y, Matsui T, Yano Y, et al. Long-term course of Crohn’s disease in Japan: incidence of complications, cumulative rate of initial surgery, and risk factors at diagnosis for initial surgery. J Gastroenterol Hepatol. 2015;30:1713–9.
Yano Y, Matsui T, Matsushima Y, et al. Time trend and risk factors of initial surgery for Crohn’s disease in Japan. J Colitis Diverticulitis. 2016;1:107. https://doi.org/10.4172/jcdc.1000107.
Uno H, Yao T, Matsui T, et al. Mortality and cause of death in Japanese patients with Crohn’s disease. Dis Colon Rectum. 2003;46:S15–21.
Oriuchi T, Hiwatashi N, Kinouchi Y, et al. Clinical course and longterm prognosis of Japanese patients with Crohn’s disease: predictive factors, rates of operation, and mortality. J Gastroenterol. 2003;38:942–53.
Wolters FL, Russel MG, Sijbrandij j, et al. Crohn’s disease: increased mortality 10 years after diagnosis in a Europe-wide population based cohort. Gut. 2006;55:510–8.
Jess T, Loftus EV Jr, Harmsen WS, et al. Survival and cause specific mortality in patients with inflammatory bowel disease: a long term outcome study in Olmsted Country, Minnesota, 1940–2004. Gut. 2006;55:1248–54.
Canavan C, Abrams KR, Mayberry JF. Meta-analysis: mortality in Crohn’s disease. Aliment Pharmacol Ther. 2007;25:861–70.
Jess T, Riis L, Vind I, et al. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis. 2007;13:481–9.
Selinger CP, Andrews J, Dent OF, et al. Cause-specific mortality and 30-year relative survival of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2013;19:1880–8.
Hovde Ø, Kempski-Monstad I, Småstuen MC, et al. Mortality and causes of death in Crohn’s disease: results from 20 years of follow-up in the IBSEN study. Gut. 2014;63:771–5.
Bitton A, Vutcovici M, Sewitch M, et al. Mortality trends in crohn’s disease and ulcerative colitis: a population-based study in Québec, Canada. Inflamm Bowel Dis. 2016;22:416–23.
Lee HS, Choe J, Kim SO, et al. Overall and cause-specific mortality in Korean patients with inflammatory bowel disease: a hospital-based cohort study. J Gastroenterol Hepatol. 2017;32:782–8.
Matsui T, Hirai F, Hisabe T. Proposed diagnostic criteria for Crohn’s disease. In: Annual of Research Group of Intractable Inflammatory Bowel Disease subsidized by the Ministry of Health, Labour, and Welfare of Japan. 2011, pp. 52–54 (in Japanese).
Ueno F, Matsui T, Matsumoto T, et al. Evidence-based clinical practice guidelines for Crohn’s disease, integrated with formal consensus of experts in Japan. J Gastroenterol. 2013;48:31–72.
Hisabe T, Hirai F, Matsui T, et al. Evaluation of diagnostic criteria for Crohn’s disease in Japan. J Gastoenterol. 2014;49:93–9.
Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19:5–36.
Kim HJ, Hann HJ, Hong SN, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm Bowel Dis. 2015;21:623–30.
Statistics bureau of the ministry of internal affairs and communications. https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00450011&kikan=00450&tstat=000001028897&cycle=7&year=20000&month=0&tclass1=000001053058&tclass2=000001053061&tclass3=000001053065&result_back=1&result_page=1&second2=1. Accessed 1 May 2018.
Prior P, Gyde S, Cooke WT, et al. Mortality in Crohn’s disease. Gastroenterology. 1981;80:307–12.
Weterman IT, Biemond I, Peña AS. Mortality and cause of death in Crohn’s disease: review of 50 years’ experience in Leiden University Hospital. Gut. 1990;31:1387–90.
Cottone M, Magliocco A, Rosselli M, et al. Mortality in patients with Crohn’s disease. Scand J Gastroenterol. 1996;31:372–5.
Farrokhyar F, Swarbrick ET, Grace RH, et al. Low mortality in ulcerative colitis and Crohn’s disease in three regional centers in England. Am J Gastroenterol. 2001;96:501–7.
Kennedy NA, Clark DN, Bauer J, et al. Nationwide linkage analysis in Scotland to assess mortality following hospital admission for Crohn’s disease: 1998–2000. Aliment Pharmacol Ther. 2012;35:142–53.
Hutfless SM, Weng X, Liu L, et al. Mortality by medication use among patients with inflammatory bowel disease, 1996–2003. Gastroenterology. 2007;133:1779–86.
Masala G, Bagnoli S, Ceroti M, et al. Divergent patterns of total and cancer mortality in ulcerative colitis and Crohn’s disease patients: the Florence IBD study 1978–2001. Gut. 2004;53:1309–13.
Watanabe K, Sasaki I, Fukushima K, et al. Long-term incidence and characteristics of intestinal failure in Crohn’s disease: a multicenter study. J Gastroenterol. 2014;49:231–8.
Howard L, Malone M. Current status of home parenteral nutrition in the United States. Transplant Proc. 1996;28:2691–5.
Loly C, Belaiche J, Louis E. Predictors of severe Crohn’s disease. Scand J Gastroenterol. 2008;43:948–54.
Limketkai BN, Parian AM, Shah ND, et al. Short bowel syndrome and intestinal failure in Crohn’s disease. Inflamm Bowel Dis. 2016;22:1209–18.
Caini S, Bagnoli S, Palli D, et al. Total and cancer mortality in a cohort of ulcerative colitis and Crohn’s disease patients: the Florence inflammatory bowel disease study, 1978–2010. Dig Liver Dis. 2016;48:1162–7.
Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1097–104.
Yano Y, Matsui T, Hirai F, et al. Cancer risk in Japanese Crohn’s disease patients: investigation of the standardized incidence ratio. J Gastroenterol Hepatol. 2013;28:1300–5.
Mizushima T, Ohno Y, Nakajima K, et al. Malignancy in Crohn’s disease: incidence and clinical characteristics in Japan. Digestion. 2010;81:265–70.
Higashi D, Katsuno H, Kimura H, et al. Current state of and problems related to cancer of the intestinal tract associated with Crohn’s disease in Japan. Anticancer Res. 2016;36:3761–6.
Sugita A, Koganei K, Tatsumi K, et al. Management of rectal cancer including cancer in the anal fistula with Crohn’s disease. Nihon Shokakibyo Gakkai Zasshi. 2013;110:396–402.
Yamamoto J, Uno H, Hirai F, et al. Clinical study of 11 cases of amyloidosis secondary to Crohn’s disease (in Japanese). Stomach Intestine. 1999;34:1255–66.
Tanimura S, Nozaki R, Ohwan T, et al. Clinical study of secondary amyloidosis in surgically treated cases of Crohn’s disease (in Japanese). J Jpn Soc Coloproctol. 2006;59:441–7.
Miyaoka M, Matsui T, Hisabe T, et al. Clinical and endoscopic features of amyloidosis secondary to Crohn’s disease: diagnostic value of duodenal observation and biopsy. Dig Endosc. 2011;23:157–65.
Sharma P, Aguilar R, Siddiqui OA, et al. Secondary systemic amyloidosis in inflammatory bowel disease: a nationwide analysis. Ann Gastroenterol. 2017;30:504–11.
Tosca Cuquerella J, Bosca-Watts MM, Anton Ausejo R, et al. Amyloidosis in inflammatory bowel disease: a systematic review of epidemiology, clinical features, and treatment. J Crohns Colitis. 2016;10:1245–53.
Lowdell CP, Shousha S, Parkins RA. The incidence of amyloidosis complicating inflammatory bowel disease. A prospective survey of 177 patients. Dis Colon Rectum. 1986;29:351–4.
Rutgeerts P, D’Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology. 1999;117:761–9.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–9.
Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–85.
Rungoe C, Langholz E, Andersson M, et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979–2011. Gut. 2014;63:1607–16.
Acknowledgements
This work was supported by the Health and Labour Sciences Research Grants for Research on Intractable Disease from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Toshiyuki Matsui received fees for lectures from Eisai Co. Ltd. and AbbVie GK. Fumihito Hirai received fees for lectures from EA Pharm Co. Ltd., Eisai Co Ltd., AbbVie GK, and Mitsubishi Tanabe Pharm Co. Ltd.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yasukawa, S., Matsui, T., Yano, Y. et al. Crohn’s disease-specific mortality: a 30-year cohort study at a tertiary referral center in Japan. J Gastroenterol 54, 42–52 (2019). https://doi.org/10.1007/s00535-018-1482-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-018-1482-y