Abstract
Lymph node (LN) metastasis from intrahepatic cholangiocarcinoma (IHCC) might be one of the most important indicators of aggressive surgical resection, yet the value of LN dissection is still controversial. To address this clinical problem, we need to better understand the multidirectional lymphatic outflow from the liver. Although most hepatic lymph flows into the hilar LNs along portal triads, there are also several lymphatic outflows directly communicating with distant areas or the general lymphatic system. Moreover, it has been revealed that LN metastasis spreads to more distal LNs through the hepatoduodenal ligament or other multidirectional lymphatic pathways connected to the general lymphatic system. Therefore, systematic LN dissection might merely be LN sampling in IHCC with LN metastasis. A multidisciplinary strategy focusing on adjuvant treatment after surgery is immediately necessary in these cases. In IHCC without LN metastasis, the accuracy of preoperative imaging assessment of LN metastasis is unsatisfactory and useless for detecting metastatic LNs in clinical settings. Therefore, prophylactic systematic LN dissection for IHCC without preoperative LN swelling is recommended for accurate LN status assessment and reduction of local recurrences. However, this procedure might not offer any clinical benefit according to the results of retrospective comparative studies. In this review, we summarize previous reports regarding lymphatic outflow of the liver and discuss LN dissection for IHCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrahepatic cholangiocarcinoma (IHCC) is the second most common primary hepatic tumor after hepatocellular carcinoma (HCC). This malignancy is a primary adenocarcinoma of the liver arising from the intrahepatic bile ducts and one of the most lethal digestive tract tumors. The incidence of IHCC has been reported to represent only about 4.1 % of primary liver carcinoma cases in Japan [1]. However, disease incidence steadily increased in both Japan and worldwide, and carries with it a high mortality rate [1–3]. Curative surgical treatment is considered the only real effective treatment [4–7], and many surgeons have recommended aggressive surgical treatment, including major hepatectomy and extended systematic lymph node (LN) dissection with or without extra hepatic bile duct resection for improving surgical outcomes [4–10]. However, to date, extended surgical treatment has not overcome IHCC malignant behavior, such as aggressive tumor spread into lymph or vascular vessels, hence the high recurrence rate, even if macroscopic curative resection is achieved [8–12].
LN metastasis, which was the most prominent malignant feature of IHCC, often occurs in either regional or distant areas, and is the greatest contributor to the negative clinical impact with much worse prognosis regardless of the induction of extended surgical treatment [13–17]. However, in a small number of patients, extended LN dissection has enhanced the long-term survival after curative surgical treatment [18]. Conversely, some investigators have suggested that patients with LN metastasis should not be considered suitable candidates for extended surgical treatment, because most LN metastases were detected at a great distance from the regional area [19–22]. Moreover, there is no evidence of the significance of LN dissection based on a definitive controlled study. Consequently, although LN metastasis might be one of the most important indicators for aggressive surgical resection, the benefit of LN dissection is still controversial. Recently, we also reported that the prognosis of patients with LN metastasis was significantly poorer regardless of LN dissection extent, and this changed our policy regarding surgical strategy for IHCC, to no routine use of the prophylactic LN dissection for patients without LN metastasis and no induction of the extended systematic removal for patients with LN metastasis [23].
Under these circumstances, in verifying the significance of LN dissection for IHCC with or without LN metastasis, we should well consider lymphatic outflow from the liver. Most aggressive surgeons have targeted regional LNs, including hilar, peripancreatic, periduodenal and gastrohepatic area, and paraaortic LNs, for extended LN dissection. However, both regional and distant LN metastases are likely to have occurred via multidirectional lymphatic outflow from the liver [24]. Although most hepatic lymph may flow into those regional lymph nodes in the hilar region along the portal triads, there are also several lymphatic outflows directly connected with distant areas of the general lymphatic system [25].
With these considerations, extended LN dissection only might be not able to regulate these lymphatic outflows, and therefore LN metastasis might have to be treated as a systemic disease. In this article, we review the lymphatic system of the liver and summarize the current knowledge of the value of LN dissection for IHCC.
Lymphatic system of the liver
The liver produces a large amount of lymphatic fluid: approximately 1–3 l/day in a normal adult liver, which represents 25–50 % of the lymphatic fluid of the entire body [26]. Hepatic lymph fluid originates from the perisinusoidal space of Disse [24, 26]. This space is located between hepatocytes and the sinusoids, and contains mainly blood plasma and also hepatic stellate cells. In this space, several substances are exchanged between sinusoidal blood and hepatocytes. This interstitial fluid in the perisinusoidal space of Disse is collected in small lymphatic capillaries along the branches of portal and hepatic vein or the hepatic capsule as hepatic lymph. These lymphatic capillaries converge to thicker lymph vessels, and drain into the first LN station or directly communicate with the general lymphatic system. In addition, the lymphatic system of the liver can be divided into deep and superficial systems [24, 27]. The deep lymphatic system lies in the portal triads and along the hepatic veins, while the superficial lymphatic system is also found on the liver surface consisting of the convex and inferior surfaces.
Deep lymphatic system
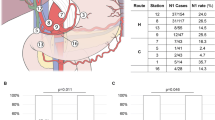
The deep lymphatic system is classified into two categories: the periportal and hepatic venous lymphatic systems. These lymphatic outflows from the liver are summarized in Fig. 1. In the periportal lymphatic system, lymphatic vessels run in Glisson’s sheath along with the portal vein, hepatic artery, or bile duct. These periportal lymphatic vessels converge to 12–15 separate vessels at the hepatic hilum [24]. This periportal hepatic lymph flows in the same direction as bile, and 80 % or more of hepatic lymph drains through this periportal lymphatic system [26, 28]. The efferent lymphatic vessels outside the liver communicate with hilar LNs and peripancreatic LNs and act as the first LN station [24, 25, 27]. Hilar LNs are connected with celiac LNs or juxtaesophageal and gastrocardiac LNs along the lesser omentum, and peripancreatic LNs reach the superior mesenteric LNs. Subsequently, the celiac and superior mesenteric routes connect with cisterna chyli through paraaortic LNs, and the juxtaesophageal route directly connects with the general lymphatic system of the posterior mediastinum [24, 27].
In the hepatic venous lymphatic system, which is another deep lymphatic system, 5–6 separate vessels leave the liver along the inferior vena cava, and hepatic lymph directly flows into the general lymphatic system of the posterior mediastinum [24, 27]. Some of the hepatic lymph traveling along the right hepatic vein flows into paraaortic LNs through the right hepatorenal ligament.
Superficial lymphatic system
The superficial lymphatic system exists in the subserosal connective tissue of the liver surface, and consists of the lymphatic vessels from the convex surface and the inferior surface drains through various routes. Most superficial lymphatic vessels directly communicate with distant LNs or the general lymphatic system. The superficial system of the liver convex surface develops along the bilateral coronary ligament, bilateral triangular ligament, and falciform ligament (Fig. 2a) [24, 27]. Their hepatic lymph directly enters the distant thoracic LNs, including pericardial, superior phrenic, and juxtaesophageal LNs across the diaphragm, and in turn flow into the general lymphatic system of the anterior mediastinum or paraaortic LNs through the right or left phrenic artery.
In the superficial system of the liver inferior surface, lymphatic vessels converge toward the hepatic hilum, and connect with the regional LNs (Fig. 2b) [24, 27, 29, 30]. In addition, lymphatics from the gall bladder flow into cystic LNs. In the back of the caudate lobe and bare area of the liver, lymphatic vessels accompany the inferior vena cava and flow into the general lymphatic system of the posterior mediastinum.
Computed tomography imaging of distant LN swelling in IHCC patients
Figure 3a shows a computed tomography (CT) image of IHCC approximately 3 cm in diameter located mainly in segment seven of the liver. This tumor invaded to the right hepatic vein, but only contacted the inferior vena cava. However, this tumor directly spread to several distant LNs of the posterior mediastinum, peri-inferior vena cava, and paraaortic area, without going through hilar LNs. In this case, the hepatic venous route of the deep lymphatic system were mainly affected the LN metastases. In a tumor located on anterior section of the liver, which infiltrated the liver surface, the distant LN swellings appeared in the pericardial and paraaortic areas, except for hilar LN swelling (Fig. 3b). The thorax and abdominal distant LN metastases passed through the convex surface of the superficial lymphatic system. The tumor, which accounted for the entire liver left lobe, also spread into the anterior and posterior mediastinum and esophagogastric LNs through the lessor omentum (Fig. 3c).
Computed tomography (CT) imaging of the distant LN swelling in patients with IHCC. a Right-sided tumor spreading directly into the mediastinum and paraaortic area through the hepatic venous lymphatic systems. b Right-sided tumor invasion into the convex surface of the liver and spreading directly into the mediastinum and paraaortic area. c Left-sided tumor spread into the mediastinum and lessor curvature
Based on these findings, lymphatic outflow from the liver is not only in the direction of the hepatoduodenal ligament, but shows multidirectional communication with regional LNs and distant LNs, including the paraaortic or thoracic area and the general lymphatic system. Moreover, normal lymphatic outflow is disrupted by either tumor infiltration of small lymphatic capillaries in the liver or LN metastatic involvement in the hepatoduodenal ligament, thereby resulting in increased hepatic lymph within the perisinusoidal space of Disse and increased backward flow into blood vessels or other lymphatic vessels, except for the hepatoduodenal ligament [31].
LN dissection in the literature
Although several prognostic factors, such as LN metastasis, multiple tumors, gross type, poor differentiation, vascular invasion, and others, have been elucidated, LN metastasis is potentially the strongest prognostic factor in IHCC, with an incidence of approximately 30–70 % [13, 32]. However, there is no consensus on systematic LN dissection for IHCC based on a definitive comparative study. All reported studies to date have been retrospective case series’ or non-controlled prospective. Therefore, no acceptable rationale for the appropriate approach for IHCC LN metastases has been established, and the value of LN dissection remains unclear. Previous studies focusing on the value of LN dissection for IHCC with or without LN metastasis summarized according to pros and cons are shown in Tables 1 and 2. In these studies, investigators describe their various opinions for LN dissection based on their results or several citations.
The value of LN dissection for IHCC with LN metastasis: pros and cons
In the pro opinion group (Table 1), most investigators have demonstrated that LN metastasis is not an independent prognostic factor, and indicated that multiple tumors is important to predict prognosis after surgical treatment compared with LN metastasis. They emphasized the presence of some long-term survivors with LN metastasis more than 3 years owing to aggressive surgical treatment, including extended LN dissection [35, 36, 43]. In fact, there are sporadic case reports of long-term survivors after extended surgical treatment for IHCC with extensive LN metastasis [57–61]. Although the aggressive surgical treatment did not clearly improve surgical outcomes even in their series, their results encouraged them to conduct further application regardless of the presence of obvious LN metastases. However, a direct comparison of surgical outcomes between patients with LN metastasis who underwent LN dissection and those who did not may be impossible in the future, because the accurate distribution of LN metastasis cannot presently be confirmed in patients who do not undergo LN dissection.
Another important topic regarding LN dissection is that the definition of regional LNs of the liver has not been elucidated. LN status was included in the staging system of IHCC in the seventh edition TNM classification, and regional LNs were also defined in this system [62]. In addition, three major abdominal routes of lymphatic spread of IHCC, including the hepatoduodenal, cardiac, and diaphragmatic routes, have been reported [63]. Nevertheless, the definition of regional LNs of the liver has not been established. Igami et al. [64], who belonged to the most aggressive surgical institution in Japan, demonstrated that gastric LN metastasis was never recognized as a single metastatic site and was accompanied by worse prognosis similar to that of paraaortic LN metastasis. They have proposed that gastric LN metastasis should be treated as distant metastasis, and thus the TNM classification for IHCC should be modified. Similarly, peripancreatic LN metastases also cause a dismal prognosis of less than 3 years in their analysis. However, no standard protocol for systematic LN dissection, which should be removed for cleaning lymphatic spread, exists to regulate the disseminated cancer cells through the multidirectional lymphatic outflow, and further studies investigating all lymphatic metastatic pathways and the first LN station of the liver are needed.
In the con opinion group (Table 2), LN metastasis was revealed to be the strongest prognostic factor, and, therefore, removal of LN metastasis cannot improve surgical outcomes, even if the removal area is extended. Furthermore, they suggested that LN metastasis was not contained in one place in the vicinity of the liver. For instance, Shimada et al. [12] demonstrated that LN metastasis spread far beyond the hepatoduodenal ligament in 87.5 % of IHCC patients with LN metastasis. Uenishi et al. [50] also showed that LN metastasis beyond the hepatoduodenal ligament was observed in 72.4 % of IHCC patients with LN metastasis, and distant LN metastasis in 23.4 % of the same patients in a multicenter study. These findings suggest that LN metastases already spread in the more distal part of LN through the hepatoduodenal ligament or the other multidirectional lymphatic pathways communicating with the general lymphatic system when they metastasize to hepatoduodenal LNs. Furthermore, Yamamoto et al. [33] could not find any significant difference in surgical outcomes between LN metastasis within the hepatoduodenal ligament and beyond the hepatoduodenal ligament, including the paraaortic area, even if macroscopic radical LN dissection was achieved. In other words, IHCC with LN metastasis actually assumes the characteristics of a systemic disorder, and therefore it is not possible to control this malignant behavior only with surgical treatment. Even some pro opinion investigators have conceded the limitations for the indication of LN dissection in IHCC with LN metastasis. Suzuki et al. [35] recommended systematic LN dissection combined with hepatectomy for mass-forming type IHCC with only a single LN metastasis. Nakagawa et al. [37] concluded that the indication for curative resection with systematic LN dissection was no more than two LN metastatic nodules. Consequently, it has been strongly suggested that this so-called systematic LN dissection is merely LN sampling under these circumstances, even if the extended systematic LN dissection had some prognostic meaning in a few previous reports regarding long-term survivors with LN metastasis.
The value of LN dissection for IHCC without LN metastasis: pros and cons
Regarding the value of LN dissection for IHCC without LN metastasis, the pro opinion group has especially remarked on the difficulty in determining LN status on the basis of preoperative imaging and the reduction of locoregional recurrence owing to prophylactic systematic LN dissection. The accuracy of preoperative imaging assessment for LN metastasis by CT scan has been unsatisfactory, and the current imaging modalities did not provide accurate LN status. It has been reported that the sensitivity and specificity of CT scan assessment for detecting LN metastases were 40–50 and 77–92 % [65–68], respectively. Recently, positron emission tomography–CT (PET–CT) has somewhat improved this accuracy; however, it remains useless for detecting metastatic LN in clinical settings [69–71]. Advances in molecular techniques have revealed that conventional histological examination cannot detect the micrometastatic LN foci in biliary carcinomas [72]. Therefore, they advocate that systematic removal of LNs might not only provide accurate staging, but also reduce the risk of local recurrences. Finally, they predicted that prophylactic systematic LN dissection has the theoretical potential to improve the long-term survival of IHCC without LN metastasis [38–40, 42, 44, 45]. Thus, routine systematic LN dissection seems to be necessary to achieve complete curative resection for IHCC, even if regional LN swelling is not observed macroscopically. However, considering the relationship between the main tumor condition and LN status, Marubashi et al. [46] suggested that systematic LN dissection can be omitted for patients with solitary lesions less than 5 cm in diameter and peripheral type IHCC, because these patients show a very low probability of LN metastasis. Similarly, Miwa et al. [38] suggested that systematic LN dissection might not be necessary in patients with mass-forming type and nodules less than 4.5 cm in diameter located in the peripheral liver for these reasons.
Furthermore, there were five studies of the direct comparison between the presence and absence of systematic LN dissection in this setting [39, 52, 53, 55, 56]. They retrospectively analyzed surgical outcomes in IHCC patients without pathological LN metastasis who underwent LN dissection and IHCC patients without the clinical LN metastasis who did not, and demonstrated no significant value of the former. On the contrary, Choi et al. [39] found that the patients without LN metastasis who underwent LN dissection showed slightly worse prognosis than patients who did not undergo LN dissection, although the difference was not statistically significant. Most recently, the largest study regarding the value of prophylactic systematic LN dissection has been reported. Kim et al. [56] revealed no difference in survival in clinically lymphadenopathy negative patients without LN dissection compared with those patients with LN dissection in 215 total cases. In their study, 51.3 % of clinical lymphadenopathy negative patients with LN dissection had pathological LN metastasis. Although the micrometastatic LN foci in the negative lymphadenopathy without LN dissection group might have a similar probability of pathological LN metastasis, the systematic removal of the micrometastases did not affect surgical outcomes. Hence, prophylactic systematic LN dissection for IHCC without preoperative LN swelling might not offer any clinical benefit based on retrospective comparative studies, although further improvement of preoperative imaging assessment for the accurate diagnosis of LN metastasis are necessary to confirm these results.
Therapeutic strategy for IHCC LN metastasis
Before March 2004, we had generally performed aggressive surgery consisting of extended hepatic lobectomy combined with systematic LN dissection including paraaortic LN for patients with IHCC. However, taking into consideration the negative clinical impact on the systematic LN dissection for IHCC, after April 2004, we altered our surgical strategy and introduced customized surgery according to tumor location, size, and apparent spread, including LN metastasis of IHCC [23]. Regarding LN dissection, we applied only extirpation of the swelling LN or the suspected metastatic LN for macroscopic curative intent. Next, we further applied the gemcitabine combined with low-dose 5-fluorouracil and cisplatin (GFP) chemotherapy as adjuvant treatment for patients with prognostic factors, including LN metastasis, intrahepatic metastasis, and positive surgical margin, as was possible. This GFP regimen consists of one 4-week course of treatment that includes a triple combination of agents consisting of gemcitabine, 5-fluorouracil and cisplatin. Gemcitabine (1000 mg/m2) was diluted with 100 ml of normal saline and administered intravenously over 30 min on days 1, 8, 15, and 22. Cisplatin at 3 mg/m2/day and 5-fluorouracil at 300 mg/m2/day were given peripherally on days 1–5, 8–12, 15–19, and 22–26, followed by 2-week withdrawal from chemotherapy [73, 74]. Induction with two cycles of GFP therapy started within at least 4 months after surgical treatment.
Figure 4 shows overall survival curves for IHCC patients with or without LN metastasis. Patients with LN metastasis showed significantly worse prognosis; the 3-year overall survival rates were 12.1 % in patients with LN metastasis, and 57.4 % in patients without LN metastasis, respectively. We investigated the value of prophylactic systematic LN dissection for patients without both clinical lymphadenopathy and pathological LN metastasis. For clinicopathological factors, the significant difference in tumor location between the D0 or sampling group and the systemic LN dissection group was observed, while there is no meaningful difference in other factors (Table 3). Consequently, prophylactic systematic LN dissection did not prolong patient prognosis after surgical treatment, and induced a worse prognosis, although some micrometastatic foci in the LNs seemed to be included in the D0 or sampling group (Fig. 5a). In addition, regarding the effect of adjuvant treatment after surgery in patients with LN metastasis, although the number of patients was small, adjuvant GFP therapy significantly improved surgical outcomes (Fig. 5b) regardless of the induction of LN dissection (Table 4).
In our experience, prophylactic systematic LN dissection does not have any value for surgical outcomes in IHCC without LN metastasis, and adjuvant treatment might have supported the better surgical outcomes in IHCC with LN metastasis. To date, no investigators have revealed the obvious efficacy of adjuvant treatment after surgery for IHCC based on a large sample or in a prospective trial. The more reliable adjuvant treatment should be established for IHCC with LN metastasis regardless of the induction of extended LN dissection.
Conclusions
According to previous reports and considering our results, prophylactic systematic LN dissection may be unnecessary in IHCC patients without LN metastasis, although improvements in preoperative imaging assessment of LN metastasis are necessary to confirm this hypothesis. Regarding IHCC with LN metastasis, considering the multidirectional lymphatic outflow from the liver or the properties of systemic disorders, we should not persist extended LN dissection. Moreover, a multidisciplinary strategy focusing on adjuvant treatment after surgery should be immediately developed.
Abbreviations
- IHCC:
-
Intrahepatic cholangiocarcinoma
- HCC:
-
Hepatocellular carcinoma
- LN:
-
Lymph node
- CT:
-
Computed tomography
- PET–CT:
-
Positron emission tomography–computed tomography
- GFP:
-
Gemcitabine combined with low-dose 5-fluorouracil and cisplatin
References
Ikai I, Arii S, Okazaki M, et al. Report of the 17th nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res. 2007;37:676–91.
Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96.
Dodson RM, Weiss MJ, Cosgrove D, et al. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg. 2013;217:736–50.
Casavila FA, Marsh JW, Iwatsuki S, et al. Hepatic resection and transplantation for peripheral cholangiocarcinoma. J Am Coll Surg. 1997;185:429–36.
Washburn WK, Lewis WD, Jenkins RL. Aggressive surgical resection for cholangiocarcinoma. Arch Surg. 1995;130:270–6.
Roayaie S, Guarrena JV, Ye MQ, et al. Aggressive surgical treatment of intrahepatic cholangiocarcinoma: predictors of outcomes. J Am Coll Sug. 1998;187:365–72.
Isaji S, Kawarada Y, Taoka H, et al. Clinicopathological features and outcome of hepatic resection for intrahepatic cholangiocarcinoma in Japan. J Hepatobiliary Pancreato Surg. 1999;6:108–16.
Kacznski J, Hanson G, Wallerstedt S. Incidence, etiologic aspects and clinicopathological features in intrahepatic cholangiocaltinoma—a study of 51 cases from a low endemicity area. Acta Oncol. 1988;m37:77–83.
Chu KM, Lai ECS, Al-Hadeede S, et al. Intrahepatic cholangiocarcinoma. World J Surg. 1997;21:301–6.
Ohtsuka M, Ito H, Kimura F, et al. Extended hepatic resection and outcomes in intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10:259–64.
Yamamoto M, Takasaki K, Yoshikawa T. Extended resection for intrahepatic cholangiocarcinoma in Japan. J Hepatobiliary Pancreato Surg. 1999;6:117–21.
Shimada M, Yamashita Y, Aishima S, et al. Hepatectomy for intrahepatic cholangiocarcinoma. Br J Cancer. 2001;88:1463–6.
Adachi T, Eguchi S. Lymph node dissection for intrahepatic cholangiocarcinoma: a critical review of the literature to date. J Hepatobiliary Pancreat Sci. 2014;21:162–8.
Ercolani G, Grazi GL, Ravaioli M, et al. The role of lymphadenectomy for liver tumors: further considerations on the appropriateness of treatment strategy. Ann Surg. 2004;239:202–9.
Guglielmi A, Ruzzenente A, Campagnaro T, et al. Patterns and prognostic significance of lymph node dissection for surgical treatment of perihilar and intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2013;17:1917–28.
Bektas H, Yeyrek C, Kleine M, et al. Surgical treatment for intrahepatic cholangiocarcinoma in Europe: a single center experience. J Hepatobiliary Pancreat Sci. 2015;22:131–7.
Ali SM, Clark CJ, Mounajjed T, et al. Model to predict survival after surgical resection of intrahepatic cholangiocarcinoma: the Mayo Clinic experience. HPB (Oxford). 2015;17:244–50.
Ohtsuka M, Ito H, Kimura F, et al. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg. 2002;89:1525–31.
Isa T, Kusano T, Shimoji H, et al. Predictive factors for long-term survival in patients with intrahepatic cholangiocarcinoma. Am J Surg. 2001;181:507–11.
DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–62.
Tamandl D, Herberger B, Gruenberger B, et al. Influence of hepatic resection margin on recurrence and survival in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2008;15:2787–94.
Clark CJ, Wood-Wentz CM, Reid-Lombardo KM, et al. Lymphadenectomy in the staging and treatment of intrahepatic cholangiocarcinoma: a population-based study using the National Cancer Institute SEER database. HPB (Oxford). 2011;13:612–20.
Morine Y, Shimada M, Utsunomiya T, et al. Clinical impact of lymph node dissection in surgery for peripheral-type intrahepatic cholangiocarcinoma. Surg Today. 2012;42:147–51.
Trutmann M, Sasse D. The lymphatics of the liver. Anat Embryol (Berl). 1994;190:201–9.
Pupulim LF, Vilgrain V, Ronot M, et al. Hepatic lymphatics: anatomy and related diseases. Abdom Imaging. 2015. doi:10.1007/s00261-015-0350-y.
Ohtani O, Ohtani Y. Lymph circulation in the liver. Anat Rec (Hoboken). 2008;291:643–52.
Kitazume N. Studies on normal architecture of subserosal lymph vessels of the human liver. Kanzo. 1983;24:581–90 (In Japanese).
Ritchie HD, Grindley JH, Bollman JL. Flow of lymph from the canine liver. Am J Physiol. 1959;196:105–9.
Turner MA, Cho SR, Messmer JM. Pitfalls in cholangiographic interpretation. Radiographics. 1987;7:1067–105.
Okuda K, Sumikoshi T, Kanda Y, et al. Hepatic lymphatics as opacified by percutaneous intrahepatic injection of contrast medium. Analysis of hepatic lymphograms in 125 cases. Radiology. 1976;120:321–6.
Chung C, Iwakiri Y. The lymphatic vascular system in liver diseases: its role in ascites formation. Clin Mol Hepatol. 2013;19:99–104.
Amini N, Ejaz A, Spolverato G, et al. Management of lymph nodes during resection of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: a systematic review. J Gastrointest Surg. 2014;18:2136–48.
Yamamoto M, Takasaki K, Yoshikawa T. Lymph node metastasis in intrahepatic cholangiocarcinoma. Jpn J Clin Oncol. 1999;29:147–50.
Weber SM, Jarnagin WR, Klimstra D, et al. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384–91.
Suzuki S, Sakaguchi T, Yokoi Y, et al. Clinicopathological prognostic factors and impact of surgical treatment of mass-forming intrahepatic cholangiocarcinoma. World J Surg. 2002;26:687–93.
Ohtsuka M, Ito H, Kimura F, et al. Extended hepatic resection and outcomes in intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10:259–64.
Nakagawa T, Kamiyama T, Kurauchi N, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2005;29:728–33.
Miwa S, Miyagawa S, Kobayashi A, et al. Predictive factors for intrahepatic cholangiocarcinoma recurrence in the liver following surgery. J Gastroenterol. 2006;41:893–900.
Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009;16:3048–56.
Ercolani G, Vetrone G, Grazi GL, et al. Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann Surg. 2010;252:107–14.
Cho SY, Park SJ, Kim SH, et al. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann Surg Oncol. 2010;17:1823–30.
de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140–5.
Saiura A, Yamamoto J, Kokudo N, et al. Intrahepatic cholangiocarcinoma: analysis of 44 consecutive resected cases including 5 cases with repeat resections. Am J Surg. 2011;201:203–8.
Ribero D, Pinna AD, Guglielmi A, et al. Surgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis of 434 patients. Arch Surg. 2012;147:1107–13.
Guglielmi A, Ruzzenente A, Campagnaro T, et al. Patterns and prognostic significance of lymph node dissection for surgical treatment of perihilar and intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2013;17:1917–28.
Marubashi S, Gotoh K, Takahashi H, et al. Prediction of the postoperative prognosis of intrahepatic cholangiocarcinoma (ICC): importance of preoperatively-determined anatomic invasion level and number of tumors. Dig Dis Sci. 2014;59:201–13.
Inoue K, Makuuchi M, Takayama T, et al. Long-term survival and prognostic factors in the surgical treatment of mass-forming type cholangiocarcinoma. Surgery. 2000;127:498–505.
Kawarada Y, Yamagiwa K, Das BC, et al. Analysis of the relationships between clinicopathologic factors and survival time in intrahepatic cholangiocarcinoma. Am J Surg. 2002;183:679–85.
Nakagohri T, Asano T, Kinoshita H, et al. Aggressive surgical resection for hilar-invasive and peripheral intrahepatic cholangiocarcinoma. World J Surg. 2003;27:289–93.
Uenishi T, Kubo S, Yamazaki O, et al. Indications for surgical treatment of intrahepatic cholangiocarcinoma with lymph node metastases. J Hepatobiliary Pancreat Surg. 2008;15:417–22.
Yamashita Y, Taketomi A, Morita K, et al. The impact of surgical treatment and poor prognostic factors for patients with intrahepatic cholangiocarcinoma: retrospective analysis of 60 patients. Anticancer Res. 2008;28:2353–9.
Shimada K, Sano T, Nara S, et al. Therapeutic value of lymph node dissection during hepatectomy in patients with intrahepatic cholangiocellular carcinoma with negative lymph node involvement. Surgery. 2009;145:411–6.
Fisher SB, Patel SH, Kooby DA, et al. Lymphovascular and perineural invasion as selection criteria for adjuvant therapy in intrahepatic cholangiocarcinoma: a multi-institution analysis. HPB (Oxford). 2012;14:514–22.
Uchiyama K, Yamamoto M, Yamaue H, et al. Impact of nodal involvement on surgical outcomes of intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2011;18(3):443–52.
Li DY, Zhang HB, Yang N, et al. Routine lymph node dissection may be not suitable for all intrahepatic cholangiocarcinoma patients: results of a monocentric series. World J Gastroenterol. 2013;19:9084–91.
Kim DH, Choi DW, Choi SH, et al. Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? A review of 17 years of experience in a tertiary institution. Surgery. 2015 [Epub ahead of print].
Yamamoto M, Takasaki K, Imaizumi T, et al. A long-term survivor of intrahepatic cholangiocarcinoma with lymph node metastasis: a case report. Jpn J Clin Oncol. 2002;32:206–9.
Uenishi T, Yamazaki O, Horii K, et al. A long-term survivor of intrahepatic cholangiocarcinoma with paraaortic lymph node metastasis. J Gastroenterol. 2006;41:391–2.
Higuchi R, Yamamoto M, Hatori T, et al. Intrahepatic cholangiocarcinoma with lymph node metastasis successfully treated by immunotherapy with CD3-activated T cells and dendritic cells after surgery: report of a case. Surg Today. 2006;36:559–62.
Murakami Y, Yokoyama T, Takesue Y, et al. Long-term survival of peripheral intrahepatic cholangiocarcinoma with metastasis to the para-aortic lymph nodes. Surgery. 2000;127:105–6.
Asakura H, Ohtsuka M, Ito H, et al. Long-term survival after extended surgical resection of intrahepatic cholangiocarcinoma with extensive lymph node metastasis. Hepatogastroenterol. 2005;52:722–4.
Sobin LH, Gospodarowicz MK, Wittekind C, editors. International Union Against Cancer (UICC). TNM classification of malignant tumours, 7th ed. Wiley-Blackwell, Oxford; 2009.
Tsuji T, Hiraoka T, Kanemitsu K, et al. Lymphatic spreading pattern of intrahepatic cholangiocarcinoma. Surgery. 2001;129:401–7.
Igami T, Ebata T, Yokoyama Y, et al. Staging of peripheral-type intrahepatic cholangiocarcinoma: appraisal of the new TNM classification and its modifications. World J Surg. 2011;35:2501–9.
Noji T, Kondo S, Hirano S, et al. CT evaluation of paraaortic lymph node metastasis in patients with biliary cancer. J Gastroenterol. 2005;40:739–43.
Park MS, Lee DK, Kim MJ, et al. Preoperative staging accuracy of multidetector row computed tomography for extrahepatic bile duct carcinoma. J Comput Assist Tomogr. 2006;30:362–7.
Grobmyer SR, Wang L, Gonen M, et al. Perihepatic lymph node assessment in patients undergoing partial hepatectomy for malignancy. Ann Surg. 2006;244:260–4.
Vilgrain V. Staging cholangiocarcinoma by imaging studies. HPB (Oxford). 2008;10:106–9.
Seo S, Hatano E, Higashi T, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts lymph node metastasis, P-glycoprotein expression, and recurrence after resection in mass-forming intrahepatic cholangiocarcinoma. Surgery. 2008;143:769–77.
Lee SW, Kim HJ, Park JH, et al. Clinical usefulness of 18F-FDG PET–CT for patients with gallbladder cancer and cholangiocarcinoma. J Gastroenterol. 2010;45:560–6.
Park TG, Yu YD, Park BJ, et al. Implication of lymph node metastasis detected on 18F-FDG PET/CT for surgical planning in patients with peripheral intrahepatic cholangiocarcinoma. Clin Nucl Med. 2014;39:1–7.
Okami J, Dohno K, Sakon M, et al. Genetic detection for micrometastasis in lymph node of biliary tract carcinoma. Clin Cancer Res. 2000;6:2326–32.
Morine Y, Shimada M, Ikegami T, et al. Usefulness of gemcitabine combined with 5-fluorouracil and cisplatin (GFP) in patients for unresectable biliary carcinoma. Hepatogastroenterol. 2009;56:307–12.
Yamashita Y, Taketomi A, Itoh S, et al. Phase II trial of gemcitabine combined with 5-fluorouracil and cisplatin (GFP) chemotherapy in patients with advanced biliary tree cancers. Jpn J Clin Oncol. 2010;40:24–8.
Acknowledgments
This study was partly supported by Japan Society for the promotion of Science (Grant-in-Aid for Scientific Research B: No. 26293288 and C: 25462091).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morine, Y., Shimada, M. The value of systematic lymph node dissection for intrahepatic cholangiocarcinoma from the viewpoint of liver lymphatics. J Gastroenterol 50, 913–927 (2015). https://doi.org/10.1007/s00535-015-1071-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-015-1071-2