Abstract
Background
Neo-adjuvant chemotherapy (CHT) has gained increasing importance in resectable and borderline resectable pancreatic cancer leading to a better performing surgery when we look at negative resection margins and selection of patients with less aggressive disease. We apply this principle to patients with Stage III (LAC) pancreatic cancer undergoing RFA and try to select patients who may benefit from a local treatment.
Methods
All patients affected by LAC were treated with RFA for a stable disease after a short CHT. Postoperative morbidity and mortality were evaluated together with overall survival (OS) and disease specific survival (DSS).
Results
We consecutively treated 57 patients affected by LAC. Median duration of CHT before RFA was 5 months. The postoperative mortality rate was zero. Overall morbidity was 14 % with RFA-related morbidity of 3.5 %. The OS and DSS were 19 months and when compared to a similar population who received RFA as up front treatment, there was no difference.
Conclusions
Our results do not support the adoption of a short CHT as a way to identify patients to treat with RFA with the most benefit. Based on this and by knowing the role of immune modulation after RFA and its specific involvement in pancreatic carcinoma, we can propose RFA as upfront treatment.
Similar content being viewed by others
Introduction
Neo-adjuvant chemotherapy (nCHT) in patients with pancreatic carcinoma has become widely accepted in early stage of disease (resectable cancer) and in borderline cancer [1, 2]. The very early systemic dissemination of pancreatic cancer [3] endorses the rationale for an up-front use of systemic therapy [4]: this has the potential to maximize the chances of a margin-negative resection and minimize the number of patients harboring aggressive disease from undergoing a fruitless surgical procedure [5]. Unfortunately, only 20 % of cases are resectable at time of diagnosis: 40 % presents with metastatic disease and the remnant 40 % with locally advanced cancer [6]. In this last group of patients an increase of resection rate is possible after nCHT as reported by different authors but results on survival rate are not homogeneous [7–11]. In our centre we defined a model of Radiofrequency ablation (RFA) in LAC [12, 13] to obtain a local control of disease. After a preliminary experience with RFA performed as upfront treatment, we observed a not negligible percentage of patients with progression of disease within three months from the procedure [14]. We, therefore, decided to set a pre-RFA systemic chemotherapy with the intent to select those patients who may benefit from a cytoreductive treatment and, on the other side, to identify those early progressive patients.
In this paper we retrospectively analyze this group of patients affected by LAC treated by RFA preceded by a short systemic chemotherapy with particular attention to morbidity and mortality rate, time to progression (TTP), overall survival (OS) and disease specific survival.
Patients and methods
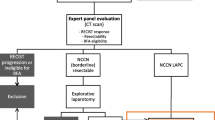
The study was approved by the local medical ethics committee. Patients with histologically proven stage III pancreatic cancer were enrolled and received the procedure following a short chemotherapy (CHT). The choice of chemotherapy scheme was up to the oncologist and this was mainly due to the variety of referral centres. Preoperative staging included contrast-enhanced computed tomography (CT) scan or magnetic resonance imaging (MRI), and serum tumour markers. For those patients who were referred from other hospitals, the MRI or CT scan were reviewed or done by our radiologists to confirm the Stage. Mandatory criteria to receive RFA after CHT was the absence of a radiological or clinical progression of disease. The inclusion and exclusion criteria and the details of the procedure have been previously described [12, 13].
Briefly, the procedure was performed under general anaesthesia and laparotomy: the ultimate confirmation of unresectability was made during surgery. A RITA® System Generator 1500X (AngioDynamics®, USA) was used. The probe (StarBurst XL, Talon or Uniblate™ depending on tumour size and shape) was placed in the tumour under US monitoring. In case of biliary duct dilation or jaundice, and/or duodenal obstruction, biliary and/or gastric by-pass was performed. Seven days after surgery, an abdominal perfusion-CT scan was performed, and the serum tumour markers were measured. Pancreatic fistula, duodenal injuries, thrombosis of the portal vein or superior mesenteric vein are considered as RFA-related complications. Follow up (consisting of clinical examination, abdominal CT scan and serum tumour marker measurement) was planned on a 3-month basis. After surgery, all patients were referred to the oncologist in order to receive chemo and/or radiotherapy (CHT-RT) when feasible.
Statistics
Descriptive statistical analyses were performed using SPSS package software 17.0 (SPSS, Chicago, III). The normally distributed continuous variables were reported as percentage, mean and standard deviation (SD). Median and interquartile ranges (IQR) were used to describe non-parametric variables. Kaplan–Meier curves were adopted to estimate the probability of survival rate at each point in time, with the censored cases being those where the expected event did not occur.
Results
We retrospectively analyze 57 patients affected by LAC diagnosed in our or other peripheral centers and prospectively enrolled between February 2007 and June 2012. Patients characteristics are shown in Table 1. Systemic chemotherapy was administered with different schemes: 54 patients received Gemcitabine-based chemotherapy, 2 patients the four drugs scheme (PEXG) and one patient was treated with Folfirinox protocol. The median duration of therapy was 5 months (IQR 4–6). In three cases palliative surgery was performed at the time of diagnosis: two double bypass and one biliary bypass. After CHT, all patients were evaluated in our centre and in all cases the locally advanced stage of disease was confirmed as stable by MRI or CT and contrast enhanced ultrasound (CEUS). The RFA was successfully conducted in all 57 patients and the postoperative course was uneventful in 86 % of cases (49 pts). Mortality rate due to the procedure was zero. Among postoperative morbidity, only 2 cases developed RFA related complications: one case of grade 1 pancreatic fistula [15] and one case of duodenal micro-perforation and severe bleeding treated conservatively (Table 2). All patients with surgery related complications had palliative surgery, but the adverse events were not associated with the palliative procedure itself other than in two cases of gastric fistula. All patients were then sent to the oncologist to complete the treatment: 84.2 % received further chemo-radiotherapy while 15.8 % of them were not able to carry on the treatment due to poor general conditions.
We observed 7 cases (12.3 %) of progression of disease within 3 months from the procedure: two hepatic progressions and 5 cases with local progression. The median time to progression (TTP) was 10 months (IQR 7.7–13.2). Among patients with a minimum follow up of 12 months (29/57), 29.8 % died from the disease, 12.2 % are alive with stable disease, 3.5 % are alive without radiological evidence of disease and 1.7 % are alive with progression. Two patients (3.5 %) died from other causes.
The median overall survival (OS) and disease specific survival were both 19 months (Figs. 1, 2). We compared this survival data to a similar population of patients affected by LAC, observed at the same time, who underwent RFA as upfront therapy and we saw that there was no difference in survival depending on the timing of RFA (p value 0.6) as shown in Fig. 3.
Discussion
We were surely surprised from the results we obtained from this study. The purpose of demonstrating that a chemotherapy done before RFA could be the key to select the patients who may benefit from a local treatment, has not been successful. The chemotherapy regimens were not the same in all patients, but they were almost all Gem-based therapies and we defined such with our oncologists, for the purpose of selection the early progressive patients with 3 months therapy, the differences between different drugs were not so important. We should not forget that RFA is a cytoreductive treatment which does not aim to eradicate the tumor: what is left in site after RFA can be specifically targeted by following radiotherapy and systemic or intra-arterial chemotherapy. These results seem in contrast to what has been recently published by Cantore and us [16] where a combined multi-treatment followed by RFA could significantly prolong survival: as Cantore himself states in his paper the extraordinary survival rate of 25.6 months may reflect the inherent selection bias that is inevitable in some patients who receive second-line RFA, who must have benefited from an earlier treatment in order to receive RFA later.
The rate of early progression was the same with or without chemotherapy (12.3 and 16 %, n.s.) and so was the survival rate (19 months): we can therefore propose RFA as an upfront treatment with no fear of neglect as a neo-adjuvant treatment? This being an observational and not randomized study, the interpretation of these data can be not univocal. Immune modulation is the other strong motivation to propose RFA [17] as up front treatment to patients with LAC: an increasing number of studies have been published regarding the role of thermal ablation in stimulating and modulating the immune system and the immune response against the tumor [18–21]. Dromi et al. [22] demonstrates in animals an increase of dendritic cell infiltration, which are the most powerful antigen presenting cells, following the ablation: subtotal RFA treatment results in systemic antitumor T cell immune responses and tumor regression.
The natural lack of dendritic cells in pancreatic carcinoma, as compared to other solid neoplasm [23], makes LAC the natural candidate for thermal ablation to obtain a systemic effect from a local treatment.
References
Sho M, Akahori T, Tanaka T, Kinoshita S, Tamamoto T, Nomi T, et al. Pathological and clinical impact of neoadjuvant chemoradiotherapy using full-dose gemcitabine and concurrent radiation for resectable pancreatic cancer. J Hepatobiliary Pancreat Sci. 2012 [Epub ahead of print].
Lee JL, Kim SC, Kim JH, Lee SS, Kim TW, do Park H, et al. Prospective efficacy and safety study of neoadjuvant gemcitabine with capecitabine combination chemotherapy for borderline-resectable or unresectable locally advanced pancreatic adenocarcinoma. Surgery. 2012;152(5):851–62. doi:10.1016/j.surg.2012.03.010.
Raut CP, Tsen JF, Sun CC, Wang H, Wolff RA, Crane CH, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60.
Belli C, Cereda S, Anand S, Reni M. Neoadjuvant therapy in resectable pancreatic cancer: A critical review. Cancer Treat Rev. 2012. doi: 10.1016/j.ctrv.2012.09.008.
Wolff RA. Neoadjuvant therapy for resectable and borderline resectable adenocarcinoma of the pancreas. Curr Drug Targets. 2012;13(6):781–8.
Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, European Study Group for Pancreatic Cancer, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358(9293):1576–85.
Strobel O, Berens V, Hinz U, Hartwig W, Hackert T, Bergmann F, et al. Resection After neoadjuvant therapy for locally advanced, “unresectable” pancreatic cancer. Surgery. 2012;152(3 Suppl 1):S33–42. doi:10.1016/j.surg.2012.05.029.
Andriulli A, Festa V, Botteri E, Valvano MR, Koch M, Bassi C, et al. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol. 2012;19(5):1644–62. doi:10.1245/s10434-011-2110-8.
Sahora K, Kuehrer I, Eisenhut A, Akan B, Koellblinger C, Goetzinger P, et al. NeoGemOx: gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery. 2011;149(3):311–20. doi:10.1016/j.surg.2010.07.048.
Laurence JM, Tran PD, Morarji K, Eslick GD, Lam VW, Sandroussi C. A systematic review and meta-analysis of survival and surgical outcomes following neoadjuvant chemoradiotherapy for pancreatic cancer. J Gastrointest Surg. 2011;15(11):2059–69. doi:10.1007/s11605-011-1659-7.
Barugola G, Partelli S, Crippa S, Capelli P, D’Onofrio M, Pederzoli P, et al. Outcomes after resection of locally advanced or borderline resectable pancreatic cancer after neoadjuvant therapy. Am J Surg. 2012;203(2):132–9. doi:10.1016/j.amjsurg.2011.03.008.
Girelli R, Frigerio I, Salvia R, Barbi E, Tinazzi Martini P, et al. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg. 2010;97:220–5.
D’Onofrio M, Barbi E, Girelli R, Martone E, Gallotti A, Salvia R, et al. Radiofrequency ablation of locally advanced pancreatic adenocarcinoma: an overview. World J Gastroenterol. 2010;16:3478–83.
Girelli R, Frigerio I, Giardino A, Regi P, Gobbo S, Malleo G, et al. Results of 100 pancreatic radiofrequency ablations in the context of a multimodal strategy for stage III ductal adenocarcinoma. Langenbecks Arch Surg. 2013;398(1):63–9. doi:10.1007/s00423-012-1011-z.
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13.
Cantore M, Girelli R, Mambrini A, Frigerio I, Boz G, Salvia R, et al. Combined modality treatment for patients with locally advanced pancreatic adenocarcinoma. Br J Surg. 2012;99(8):1083–8. doi:10.1002/bjs.8789.
Haen SP, Pereira PL, Salih HR, Rammensee HG, Gouttefangeas C. More than just tumor destruction: immunomodulation by thermal ablation of cancer. Clin Dev Immunol. 2011. doi:10.1155/2011/160250.
Waitz R, Solomon SB. Can local radiofrequency ablation of tumours generate systemic immunity against metastatic disease? Radiology. 2009;251(1):1–2.
Dallal RM, Christakos P, Lee K, Egawa S, Son Y, Lotse MT. Paucity of dendritic cells in pancreatic cancer. Surgery. 2002;131:135–8.
Li-Song T, Ke-Tao J, Na H, Jiang C. Radiofrequency ablation, heat shock protein 70 and potential anti-tumour immunity in hepatic and pancreatic cancers: a mini review. Hepatobiliary Pancreat Dis Int. 2010;9(4):361–5.
Muerkoster S, Wegehenkel K, Artl A, Witt M, Sipos B, Kruse ML, et al. Tumour stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1 beta. Cancer Res. 2004;64:1331–7.
Dromi SA, Walsh MP, Herby S, Traughber B, Xie J, Sharma KV, Sekhar KP, et al. Radiofrequency ablation induces antigen presenting cell infiltration and amplification of weak tumour induced immunity. Radiology. 2009;251(1):58–66.
Rovere-Querini P, Manfredi A. Tumour destruction and in situ delivery of Antigen Presenting Cells promote anti-neoplastic immune responses: implications for the immunotherapy of pancreatic cancer. JOP. 2004;5(4):308–14.
Conflict of interest
The authors declare that they do not have conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Frigerio, I., Girelli, R., Giardino, A. et al. Short term chemotherapy followed by radiofrequency ablation in stage III pancreatic cancer: results from a single center. J Hepatobiliary Pancreat Sci 20, 574–577 (2013). https://doi.org/10.1007/s00534-013-0613-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-013-0613-3