Abstract
Background/purpose
Cytomegalovirus (CMV) infection remains a challenge following liver transplantation. Preemptive treatment is an effective strategy for CMV infection. However, how long preemptive treatment should be applied is not defined.
Methods
Clinical records of preemptive treatment for CMV infection in patients who underwent liver transplantation were collected. CMV antigenemia (pp65) was monitored weekly during hospital stay and subsequently on follow up whenever indicated clinically. Antiviral treatment was administered based on positive antigenemia (>1 positive cell per 500,000 leukocytes) and discontinued when antigenemia became negative.
Results
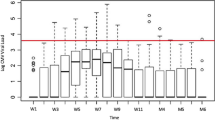
CMV infection was diagnosed in 58 (43.9%) of 132 liver transplantation patients. All 58 patients were seropositive for CMV before transplantation. CMV infection was first diagnosed at a median time of 20 days (interquartile range [IQR] 15.3–26) after transplantation. Twelve (20.7%) patients developed repeated infections. Only one of 58 patients (1.7%) was suspected to have invasive disease. The median (IQR) duration of antiviral treatment was 7 (7–12) days. Of these patients with CMV infection, 14 (24.1%) patients developed acute rejection peri-anti-CMV treatment and 36 (62.1%) developed other infectious complications.
Conclusion
Preemptive treatment is an effective way to halt the progression of asymptomatic CMV infection. A brief course of antiviral treatment is enough for seropositive patients with CMV infection after liver transplantation.


Similar content being viewed by others
References
Kanj SS, Sharma AI, Clavien PA, et al. Cytomegalovirus infection following liver transplantation: review of the literature. Clin Infect Dis. 1996;22:537–49.
Rubin RH. Cytomegalovirus infection in the liver transplant recipient. Epidemiology, pathogenesis, and clinical management. Clin Liver Dis. 1997;1:439–52.
Evans PC, Soin A, Wreghitt TG, et al. An association between cytomegalovirus infection and chronic rejection after liver transplantation. Transplantation. 2000;69:30–5.
Nebbia G, Mattes FM, Cholongitas E, et al. Exploring the bidirectional interactions between human cytomegalovirus and hepatitis C virus replication after liver transplantation. Liver Transpl. 2007;13:130–5.
Sun H-Y, Wagener MM, Singh N. Prevention of posttransplant cytomegalovirus disease and related outcomes with valganciclovir: a systematic review. Am J Transplant. 2008;8:2111–8.
Razonable RR. Cytomegalovirus infection after liver transplantation: current concepts and challenges. World J Gastroenterol. 2008;14:4849–60.
Munksgaard B. Cytomegalovirus. Am J Transplant. 2004;4(Suppl 10):51–8.
Small LN, Lau J, Snydman DR. Preventing post-organ transplantation cytomegalovirus disease with ganciclovir: a meta-analysis comparing prophylactic and preemptive therapies. Clin Infect Dis. 2006;43:869–80.
Sagedal S, Rollag H, Hartmann A. Cytomegalovirus infection in renal transplant recipients is associated with impaired survival irrespective of expected mortality risk. Clin Transplant. 2007;21:309–13.
Limaye A, Bakthavatsalam R, Kim HW, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006;81:1645–52.
Borchers AT, Perez R, Kaysen G, et al. Role of cytomegalovirus infection in allograft rejection: a review of possible mechanisms. Transplant Immunol. 1999;7:75–82.
Hoppe L, Marroni CA, Bressane R, et al. Risk factors associated with cytomegalovirus infection in orthotopic liver transplantation. Transplant Proc. 2006;38:1922–3.
de Jong MD, Galasso GJ, Gazzard B, et al. Summary of the II International Symposium On Cytomegalovirus. Antiviral Res. 1998;39:141–62.
Rubin RH. Preemptive therapy in immunocompromised hosts. N Engl J Med. 1991;324:1057.
Kusne S, Grossi P, Irish W, et al. Cytomegalovirus pp65 antigenemia monitoring as a guide for preemptive therapy: a cost effective strategy for prevention of cytomegalovirus disease in adult liver transplant recipients. Transplantation. 1999;68:1125–31.
Singh N, Wannstedt C, Keyes L, et al. Indirect outcomes associated with cytomegalovirus (opportunistic infections, hepatitis C virus sequelae, and mortality) in liver transplant recipients with the use of preemptive therapy for 13 years. Transplantation. 2005;79:1428–34.
Singh N, Wannstedt C, Keyes L, et al. Valganciclovir as preemptive therapy for cytomegalovirus in cytomegalovirus-seronegative liver transplant recipients of cytomegalovirus-seropositive donor allografts. Liver Transpl. 2008;14:240–4.
Khoury JA, Storch GA, Bohl DL, et al. Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am J Transplant. 2006;6:2134–43.
Fishman JA, Emery V, Freeman R, et al. Cytomegalovirus in transplantation—challenging the status quo. Clin Transplant. 2007;21:149–58.
Slifkin M, Ruthazer R, Freeman R, et al. Impact of cytomegalovirus prophylaxis on rejection following orthotopic liver transplantation. Liver Transpl. 2005;11:1597–602.
Eid AJ, Razonable RR. Cytomegalovirus disease in solid organ transplant recipients: advances lead to new challenges and opportunities. Curr Opin Organ Transplant. 2007;12:610–7.
Opelz G, Dohler B, Ruhenstroth A. Cytomegalovirus prophylaxis and graft outcome in solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:928–36.
Levitsky J, Singh N, Wagener MM, et al. A survey of CMV prevention strategies after liver transplantation. Am J Transplant. 2008;8:158–61.
Lopau K, Greser A, Wanner C. Efficacy and safety of preemptive anti-CMV therapy with valganciclovir after kidney transplantation. Clin Transplant. 2007;21:80–5.
Salerno F, Merli M, Cazzaniga M, et al. MELD score is better than Child-Pugh score in predicting 3-month survival of patients undergoing transjugular intrahepatic portosystemic shunt. J Hepatol. 2002;36:494–500.
Wiesner R, Edwards E, Freeman R, et al. The United Network For Organ Sharing Liver Disease Severity Score Committee. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–6.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Dahiya and C.-F. Lee contributed equally to this study.
About this article
Cite this article
Dahiya, D., Lee, CF., Chan, KM. et al. A short-term preemptive treatment for cytomegalovirus infection in seropositive patients after liver transplantation. J Hepatobiliary Pancreat Sci 18, 32–38 (2011). https://doi.org/10.1007/s00534-010-0286-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-010-0286-0