Abstract
Purpose
To systematically review the proportion and incidence of CVAD-associated complications in pediatric patients with cancer.
Methods
PubMed, Embase, and the Cumulative Index of Nursing and Allied Health Literature were searched from 2012 to 2022. Cohort studies and the control arm of randomized controlled trials, which reported CVAD-associated complications in pediatric patients aged 0–18 years, were included. CVAD complications were defined as CVAD failure, central line–associated bloodstream infection (CLABSI), local infection, occlusion, CVAD-associated venous thromboembolism, dislodgement/migration, breakage/rupture, and dehiscence. The pooled proportion and incidence rate (IR) for each CVAD-associated complication were reported.
Results
Of 40 included studies, there was mixed quality of methods and reporting. Approximately 31.4% (95% confidence interval [CI] 22.5–41.1; 6920 devices) of devices experienced a CVAD-associated complication, and 14.8% (95% CI 10.2–20.1; 24 studies; 11,762 devices) of CVADs failed before treatment completion (incidence rate (IR) of 0.5 per 1000 catheter days (95% CI 0.3–0.8; 12 studies; 798,000 catheter days)). Overall, 21.2% (95% CI 14.3–28.9; 26 studies; 5054 devices) of CVADs developed a CLABSI, with an IR of 0.9 per 1000 catheter days (95% CI 0.6–1.3; 12 studies; 798,094 catheter days). Tunneled central venous catheters (TCVC) and peripherally inserted central catheters (PICCs) were associated with increased complications in comparison to totally implanted venous access devices (TIVADs).
Conclusion
CVAD complication rates in this population remain high. TCVCs and PICCs are associated with increased complications relative to TIVADs. Insufficient evidence exists to guide device selection in this cohort, necessitating further research to determine the role of PICCs in pediatric cancer care.
PROSPERO: CRD42022359467.
Date of registration: 22 September 2022.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, an estimated 400,000 children and adolescents (0–19 years) are diagnosed with cancer annually [1]. Central venous access devices (CVADs) are essential in facilitating anti-cancer and supportive therapies. CVAD type and timing of insertion vary greatly and are influenced by multiple factors including individual patient circumstances, cancer type, treatment required, proceduralist availability (i.e., surgeon, anesthetist, or nurse practitioner), and clinician preference. The chosen device may include a peripherally inserted central catheter (PICC), totally implantable venous access device (TIVAD), or tunneled central venous catheter (TCVC) [2].
General pediatric data indicates that one in four patients with CVADs will experience a significant complication or device failure before completing therapy [3, 4]. Post-insertion complications include central line–associated bloodstream infection (CLABSI), CVAD-associated venous thromboembolism (VTE), local infection, mechanical complications, and dehiscence [4, 5]. These complications require treatment, resulting in delays to anti-cancer therapy and thus increasing morbidity and mortality [3,4,5,6,7]. Oncology patients are a distinct cohort whose susceptibility to complications is unique secondary to the cancer itself and treatments administered. Understanding the incidence of CVAD-associated complications in this cohort is important to help guide decision-making on device selection and where further research is needed to reduce adverse events.
This systematic review aimed to determine the current evidence regarding the incidence of CVAD-associated complications in pediatric patients with cancer.
Methods
This study used standard methods for systematic reviews and is reported in accordance with the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement” [8] and the Meta-Analyses of Observational Studies in Epidemiology (MOOSE) checklist [9]. This study was registered with PROSPERO on 22 September 2022 (CRD42022359467).
Eligibility criteria
A systematic search (see Online Resource 1) was conducted to look for studies examining incidence, failure, and/or post-insertion complications of CVADs in pediatric oncology patients. Procedural/insertion complications were not included. Studies were eligible for inclusion if they met the following criteria: (1) cohort design (prospective or retrospective) or (2) control arm of randomized controlled trials (RCTs), (3) failure and/or complications of CVADs included as an outcome measure, (4) pediatric patients aged 0 to 18 years, (5) patients with an oncological diagnosis, and (6) CVAD inserted for any length of time during their treatment. Studies were excluded if they were not written in English and/or if they were published prior to 2012 to best reflect current practices.
Outcome measures
The primary outcome was CVAD complications as a composite (overall complications). The secondary outcomes were CVAD complications post-successful CVAD insertion:
- 1.
-
2.
CLABSI [11]
-
3.
Local CVAD infection [12]
-
4.
Occlusion [13]
-
5.
CVAD-associated VTE [13]
-
6.
Dislodgement or migration [13]
-
7.
Breakage and/or rupture [14]
-
8.
Dehiscence [15]
Full description of definitions is available in Online Resource 2.
Search strategy and study selection
The US National Library of Medicine National Institutes of Health (PubMed), Embase, and Cumulative Index of Nursing and Allied Health Literature (CINAHL) were systematically searched. Medical subject headings and searches were developed in conjunction with a healthcare librarian (see Online Resource 1) and screened for inclusion independently by two authors using Covidence [16]. References of full-text articles were reviewed to identify additional studies. Disagreements were resolved through review by a third author.
Data extraction and missing data
Data extraction was performed by one reviewer using a standardized data extraction form, checked by a second author. The data fields extracted included country, study design, population, diagnosis, catheter type, frequency of CVAD failure and/or complications, catheter days, and CVAD risk factors. Where data were missing, study authors were contacted.
Statistical methods
The proportion and corresponding 95% confidence interval (CI) of the different complications (overall complications, failure, CLABSI, local infection, VTE, occlusion, dehiscence, dislodgement/migration, and breakage/rupture) were calculated using a random effects model to consider effects from small studies. Where studies reported catheter days, the incidence rate (IR) and corresponding 95% CI were calculated as events per 1000 catheter days. The point estimates (with 95% CI’s) from separate datasets were pooled using the DerSimonian-Laird random effects method [17], with the variances of the raw proportions stabilized using the Freeman-Turkey double arcsine transformation [18, 19]. The prediction interval is also reported to reflect the uncertainty expected in the summary effect if a new study is included in the meta-analysis [20]. Between-study variations were assessed using (1) the Chi-square (Chi2) test of heterogeneity to evaluate whether the variation between studies exceeded that expected by chance, whereby p ≤ 0.01 indicated the presence of heterogeneity, and (2) the Higgins I2 statistic, to estimate the percentage of the total variation in effect estimates across the studies attributable to heterogeneity rather than chance [21]. Publication bias was examined using Funnel plots and Egger’s test. These analyses were conducted using R (version 4.2.3) [22].
Subgroup analyses
Subgroup analysis was performed on CVAD type where data were available. There was insufficient data to subgroup by cancer diagnosis (solid tumor versus hematological malignancy) as originally planned.
Risk of bias assessment
The Mixed Methods Appraisal Tool (MMAT) [23] was used by two reviewers to assess the quality of evidence for the studies included. Disagreements in rating were discussed with a third reviewer.
Results
Systematic search results
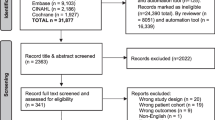
Figure 1 demonstrates the study selection process in accordance with the PRISMA guidelines [8]. A total of 382 studies were identified in the initial screening, and 40 studies met the inclusion criteria.
Characteristics of included studies
Study characteristics are summarized in Table 1.. Of the 40 studies included, there were 29 (72.5%) retrospective cohort (RC) studies, 7 (17.5%) prospective cohort (PC) studies, 1 (2.5%) retrospective case–control (RCC) study, and 3 (7.5%) randomized control trials (RCT, control arm only). Fourteen (35%) studies included hematological malignancy only [24,25,26,27,28,29,30,31,32,33,34,35,36], 1 (2.5%) study included solid tumor malignancy only [37], and the remaining 25 (62.5%) studies included all malignancies [5, 24, 38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. Studies were conducted across Asia and Pacific [40, 43, 45, 48, 51], North America [28, 30, 34, 38, 47, 53, 58, 61, 62], South America [46], Africa [60], and Europe [5, 24,25,26, 29, 31,32,33, 36, 37, 39, 41, 42, 44, 49, 50, 52, 54,55,56,57, 59, 63].
Study quality
The MMAT tool [23] was used to assess the quality of the studies, and overall, the quality of the studies included was mixed, as summarized in Table 2. There were several studies that did not provide adequate definitions for outcomes. Redkar et al. [43] were contacted and able to provide a definition for CLABSI but not for other complications; therefore, only data for CLABSI were included in the analysis. Only device failure data was able to be included for both Buonpane et al. [53] and Mangum et al. [47] as other definitions were unable to be clarified by the authors. Three studies grouped CLABSI and local infection together, and as these were not able to be clarified, data from these outcomes were not included [24, 36, 38]. One study did not meet the criteria for CLABSI and was excluded [25]. Several studies were unable to clarify if patients with CVAD-associated VTE were symptomatic and thus were excluded [5, 34, 35, 38, 48].
Outcomes
Table 3 reports the pooled proportions and IRs of CVAD-associated complications.
Overall complications
Overall, 31.4% (95% CI 22.5–41.1; 33 studies, 6920 devices) of pediatric CVADs experienced a complication, with an IR of 2.3 per 1000 catheter days (95% CI 1.6–3.2; 14 studies; 840,688 catheter days; Table 3). There was a high degree of heterogeneity observed in the pooled data for both proportion (I2 = 99%; Chi2 = 2399, df = 32, p = < 0.01, prediction interval [0.0–88.9]) and IR (I2 = 98%; Chi2 = 680.8, df = 13, p = < 0.01, prediction interval [0.2–6.8]). Tunneled CVADs had the highest pooled proportion of overall complications (TCVC 33.1% [95% CI 23.0–44.0], 1092 CVADs; TCCVC 37.5% [95% CI 32.0–43.4]; 293 CVADs) and the highest pooled IR per 1000 catheter days (1.8 [95% CI 1.5–2.1], 137,890 catheter days). PICCs had the lowest pooled proportion of overall complications (22.7% [95% CI 2.9–52.6]; 358 CVADs), whereas TIVADs had the lowest pooled IR per 1000 catheter days (1.3 [95% CI 0.9–1.8]; 267,898 catheter days). Funnel plots for both proportions and rates were visually symmetrical (Egger’s test: p = 0.19 and 0.12, respectively).
CVAD failure
Overall, 14.8% (95% CI 10.2–20.1; 24 studies; 11,762 devices; I2 = 98%; Chi2 = 943, df = 23, p = < 0.01, prediction interval [0.0–48.8]) of CVADs failed prior to completion of planned therapy, with an IR of 0.5 per 1000 catheter days (95% CI 0.3–0.8; 12 studies; 798,000 catheter days (I2 = 96%; Chi2 = 290, df = 11, p = < 0.01, prediction interval [0.0–2.2]; Table 3). Tunneled CVADs had the highest pooled proportion of device failure (TCVC 21.2% [95% CI 4.1–46.2], 1136 CVADs; TCCVC 30.4% [95% CI 25.2–36.0]; 293 CVADs). TIVADs had the highest pooled IR per 1000 catheter days of device failure (0.2 [95% CI 0.0–0.8]; 260,635 catheter days). Funnel plot for proportion was asymmetrical on visual inspection (p = 0.04), but symmetrical for rates (p = 0.59).
CLABSI
Overall, 21.2% (95% CI 14.3–28.9; 26 studies; 5052 devices; I2 = 98%; Chi2 = 1031, df = 25, p = < 0.01, prediction interval [0.0–67.7]) of CVADs developed a CLABSI, with an IR of 0.9 per 1000 catheter days (95% CI 0.6–1.3; 12 studies; 798,094 catheter days; I2 = 96%; Chi2 = 267, df = 11, p = < 0.01, prediction interval [0.0–2.7]; Table 3). Figure 2 demonstrates the pooled proportion by device type. TCVCs had the highest pooled proportion of CLABSI (30.4% [95% CI 21.3–40.3]; 785 CVADs) and the highest pooled IR per 1000 catheter days (1.7 [95% CI 1.4–1.9]; 99,681 catheter days). Funnel plot was symmetrical on visual inspection for both proportions and rates (p = 0.13 and 0.55, respectively).
Pooled proportion of CLABSI by device subgroup. Abbreviations: CLABSI, central line–associated bloodstream infection; TCVC, tunneled central venous catheter; TIVAD, totally implanted venous access device; PICC, peripherally inserted central catheter; TCCVC, tunneled cuffed central venous catheter; CI, confidence interval
CVAD-associated VTE
Overall, 5.2% (95% CI 2.2–9.3; 12 studies; 4008 devices; I2 = 95%; Chi2 = 244, df = 11, p = < 0.01, prediction interval [0.0–25.6]) of CVADs developed a VTE. The IR of VTE per 1000 catheter days was 0.0 (95% CI 0.0–0.1; 3 studies, 147,455 catheter days; I2 = 22%; Chi2 = 2.57, df = 2, p = 0.28, prediction interval [0.0–1.3]; Table 3). TIVADs had the highest pooled proportion of VTE (37.2% [95% CI 28.6–46.4]; 121 devices). No studies reported catheter days by device type; therefore, IRs for subgroup analysis could not be determined. Funnel plot was symmetrical on visual inspection for both proportions and rates (p = 0.14 and 0.14, respectively).
Local infection
Overall, 3.9% (95% CI 1.5–7.1; 11 studies; 2497 devices; I2 = 92%; Chi2 = 120, df = 10, p = < 0.01, prediction interval [0.0–20.4]) of CVADs experienced local infection, with an IR of 0.1 per 1000 catheter days (95% CI 0.0–0.3; 9 studies; 737,729 catheter days; I2 = 93%; Chi2 = 108, df = 8, p = < 0.01; prediction interval [0.0–0.8]; Table 3). TIVADs had the highest pooled proportion of local infection (7.3% [95% CI 3.9–11.7); 501 CVADs) and the highest pooled IR per 1000 catheter days (0.1 [95% CI 0.1–0.2]; 260,635 catheter days). Funnel plot was symmetrical on visual inspection for both proportions and rates (p = 0.47 and 0.45, respectively).
Occlusion
As shown in Table 3, overall, 6.3% (95% CI 3.8–9.3; 11 studies; 2562 devices; (I2 = 88%; Chi2 = 83.15, df = 10, p = < 0.01; prediction interval [0.0–20.3]) of CVADs experienced occlusion. The IR of occlusion per 1000 catheter days was 0.8 (95% CI 0.3–1.3; 7 studies, 513,716 catheter days; (I2 = 97%; Chi2 = 202, df = 6, p = < 0.01; prediction interval [0.0–3.5]). PICCs had the highest pooled proportion of occlusion (7.3% [95% CI 4.9–10.6]; 354 CVADs). TCVCs had the highest pooled IR per 1000 catheter days of occlusion (0.4 [95% CI 0.2–0.6]; 38,209 catheter days); although no studies reported catheter days for PICCs, therefore, IRs for PICCs could not be determined. Funnel plot was symmetrical on visual inspection for proportion (p = 0.16) but asymmetrical on visual inspection for rates (p < 0.01).
Dislodgement/migration
Overall, 4.6% (95% CI 2.6–7.1; 12 studies; 2785 devices; I2 = 87%; Chi2 = 83, df = 11, p = < 0.01; prediction interval [0.0–17.0]) of CVADs experienced dislodgement/migration, with an IR of 0.2 per 1000 catheter days (95% CI 0.1–0.4; 9 studies; 605,885 catheter days; I2 = 92%; Chi2 = 102, df = 12, p = < 0.01; prediction interval [0.0–1.1]; Table 3). TCVCs had the highest pooled proportion of dislodgement/migration (8.0% [95% CI 2.2–16.4]; 436 CVADs) and the highest pooled IR per 1000 catheter days (1.2 [95% CI 0.8–1.5]; 38,209 catheter days). Funnel plot was symmetrical on visual inspection for both proportions and rates (p = 0.27 and 0.28, respectively).
Breakage/rupture
As shown in Table 3, overall, 1.7% (95% CI 0.5–3.5; 9 studies; 2002 devices; I2 = 83%; Chi2 = 46.2, df = 8, p = < 0.01; prediction interval [0.0–10.3]) of CVADs experienced breakage/rupture. The IR of breakage/rupture per 1000 catheter days was 0.1 (95% CI 0.0–0.3; 6 studies, 379,447 catheter days; I2 = 93%; Chi2 = 69, df = 5, p = < 0.01; prediction interval [0.0–1.2]). TCVCs had the highest pooled proportion of breakage/rupture (3.1% [95% CI 0.0–11.1]; 163 CVADs). No studies reported catheter days by device type; therefore, IRs for device types could not be determined. Funnel plots were symmetrical on visual inspection for both proportions and rates (p = 0.93 and 0.43, respectively).
Dehiscence
Dehiscence was only reported in one study [36]. The proportion of dehiscence in this study was 2.4% (95% CI 0.0–12.7; 1 study; 322 devices; Table 3). IR was unable to be calculated as catheter days were not reported. TIVADs had the highest proportion of dehiscence (6.7% [95% CI 3.9–10.5]; 254 CVADs).
Discussion
This systematic review and meta-analysis is the first to provide a comprehensive overview of CVAD-associated complications in pediatric patients with cancer, incorporating data from 40 studies.
This meta-analysis demonstrated that overall, 31.4% (95% CI 22.5–41.1; 6920 devices) of CVADs experienced a complication, with 14.8% (95% CI 10.2–20.1; 24 studies; 11,762 devices) failing prior to treatment completion. Although no contemporary systematic reviews or meta-analysis data on CVAD complications in pediatric patients with cancer is available for comparison, these values are consistent with those reported in pediatric cohorts, where 20–30% of patients will experience a significant device complication [4]. Additionally, these results are also consistent with historical data from a 2005 prospective study by Fratino et al. [13] who reported 40% of pediatric patients with cancer experienced at least one complication of their CVAD. CVAD complications have significant burdens on the patient and healthcare system, leading to unplanned hospital admissions, additional treatments (e.g., antibiotics, anti-coagulants), and additional procedures (device replacement). Such interventions cause distress for caregivers and patients, costs to the healthcare system, and treatment delays and more recently have been linked with increased morbidity and mortality [3, 7]. A study by Athale et al. [7] found that after adjusting for age, sex, diagnostic era, and cancer type, CVAD dysfunction was an independent determinant of 5-year overall survival (OS) (HR 1.87; 95% CI 1.02–3.42, p = 0.043) and event-free survival (EFS) (HR1.96; 95% CI 1.23–3.41, p = 0.018) in pediatric patients with cancer. It is important to note that the “overall” complication rate in our study should be interpreted in the context of significant heterogeneity with study design, diagnosis, and complications reported. However, even with considering the study heterogeneity, the data shows that a large proportion of these patients are experiencing complications. Further prospective studies are needed to determine the etiology of CVAD dysfunction and interventions which can be implemented to reduce the morbidity and mortality associated with CVAD use in these patients.
Outside of “overall” complications, the largest amount of data in pediatric patients with cancer was in relation to CLABSI. Our data found CLABSI occurred at an IR of 0.9 per 1000 catheter days (95% CI 0.6–1.3). The CLABSI rate in the literature for pediatric oncology patients is reported as being 2.1 per 1000 catheter days [13]. There are several reasons this meta-analysis found a lower IR of CLABSI compared with previously reported data. Firstly, there have been significant changes in CVAD care practices that have been strongly driven to reduce infection associated with these devices [64]. Secondly, data in this meta-analysis is representative of “all malignancy,” and this number may change when separating hematological from solid tumor malignancy, which have historically had higher rates of infection secondary to their underlying disease process and associated treatments [5, 49, 52]. Subgroup analysis by malignancy type was unable to be conducted on this data secondary to insufficient studies reporting CVAD complications by malignancy type. Thirdly, as already highlighted, this meta-analysis had minimal data in relation to PICCs, including no catheter days relating to CLABSI for PICCs despite PICCs being an increasingly utilized device in pediatric cancer care. It is known that CVAD dysfunction, e.g., occlusion and VTE, is associated with increased rates of infection [7] and without having sufficient data on PICCs and their complications, our CLABSI rate may be underrepresented. CLABSI results in increased healthcare costs and burden on patients and families with prolonged hospitalization, antibiotic treatment, and potential removal and replacement of their device [13]. CLABSI remains a significant burden to this population, and as our CVAD type and use have expanded, we also need to expand our understanding of this complication across all devices to help guide decision-making on device selection and infection prevention.
This review and meta-analysis also sought to understand CVAD-associated complications specific to device subgroups. Overall, TCVCs had the highest proportion of overall complications (33.1%, 95% CI 23.0–44.0), device failure (TCCVCs 30.4%, 95% CI 25.2–36.0; TCVC 21.2%, 95% CI 4.1–46.2), CLABSI (30.4%, 95% CI 21.3–40.3), dislodgement/migration (8%, 95% CI 2.2–16.4), and breakage/rupture (3.1%, 95% CI 0.0–11.1). It appeared that TIVADs, with the exception of CVAD-associated VTE and local infection, were associated with the lowest proportion of complications. There was less published data on PICCs, but PICCs had the highest proportion of occlusion (7.3%, 95% CI 4.9–10.6). The data on IRs by device type are less reliable as only 15 studies (37.5%) reported catheter days, and thus, it is difficult to draw meaningful conclusions from these results. Consistent with our data, though not specific to the oncology population, a systematic review in pediatric patients by Ullman et al. [4] found that TCVCs and PICCs were associated with a higher proportion of overall complications for failure, CLABSI, occlusion, and dislodgement/migration relative to TIVADs. A recent study was published to provide guidance on appropriate device selection in pediatric patients titled The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics: miniMAGIC [2]. The panel recommended the use of either TCVCs or TIVADs (if > 10 kg) as appropriate in pediatric patients with cancer. The decision between a TIVAD and a TCVC for therapy is determined by the treating team and is based on the diagnosis and therapy required. Over time, PICCs have emerged as an additional device for the delivery of anti-cancer therapy in this cohort. The use of PICCs across all ages was rated by the miniMAGIC panel as uncertain due to concerns relating to procedural and post-insertion complications such as infection and thrombosis [2]. Whilst the panel deemed it appropriate to place a PICC to commence urgent therapy for cancer (not compatible with a peripheral device), the appropriateness of doing this routinely was uncertain [2]. The available data from this systematic review and meta-analysis highlights the limited studies available on PICCs in this cohort and the lack of evidence to guide device decision-making in this population. It is also evident from this review that there is huge clinical and statistical heterogeneity in this cohort, and careful decision-making is needed when selecting devices, which is currently being driven by health service availability and preference and not necessarily on evidence. Further prospective research is needed comparing the use of and complications of PICCs in comparison with TCVCs and TIVADs to determine their role in this cohort.
This review highlighted the lack of good quality evidence surrounding CVAD-associated complications in pediatric patients with cancer. This lack of evidence is twofold; firstly, there is limited data available looking at CVAD complications within this specific cohort. As a result, the data that is available is mostly retrospective and confounded by many factors, such as the inability to subgroup by diagnosis or treatment, the inability to subgroup by device type across all studies, and differences in CVAD care and maintenance. This will likely improve in time; there is a trend of increasing numbers of CVAD research in this cohort over time, with 65% of the included studies in this review being conducted within the last 5 years. Secondly, there are significant issues with the accurate collection and reporting of vascular access data in these patients. As highlighted throughout this review, several studies had to be excluded or only limited data included as a result of poor outcome definition and/or incomplete data collection. In addition, our definitions (see online resource 2 – outcome definitions) had to be kept broad to enable us to capture the current scope of literature in this field. In 2021, Schults et al. [65] proposed an international consensus on the minimum details of reporting on vascular access research, including patient demographics, device characteristics, insertion details, CVAD care, and complications. Future research in this field needs to hold researchers accountable to the minimum standard of vascular reporting to enable us to accurately understand the incidence of CVAD-associated complications and why they are occurring.
Limitations
The results of this systematic review and meta-analysis should be interpreted in the context of its limitations. Firstly, only 37.5% of studies reported catheter days, which limited the number of studies that could be included in the meta-analysis. Secondly, there was significant heterogeneity in the study populations utilized. 62.5% of studies included “all” malignancies. Hematological and solid tumor malignancies in pediatrics have significant differences not only in the pathophysiology of the disease but also in the chemotherapy and treatment administered, making generalizability difficult. There is also inconsistency internationally around the standard of care for vascular device maintenance. Improved and accurate reporting will help to address this issue. Due to the small numbers of reporting, subgroup analyses were difficult, making attempts to reduce heterogeneity difficult. The heterogeneity of this cohort was also reflected in the level of statistical heterogeneity seen in the analyses. Thirdly, whilst an attempt to keep the data relevant to modern CVAD care was made by limiting studies to the last 10 years, practices for CVAD care have evolved, and there is a risk that the pooled data may not accurately represent current rates of complications. We also elected to only include studies published in English, and there is a risk that as a result some data in this field may be missed. Finally, whilst our study reports associations between CVAD devices and complications, these do not reflect causation.
Conclusions
The results from this systematic review and meta-analysis highlight the paucity of good quality evidence on CVAD-associated complications in pediatric patients with cancer. What is clear from the results available is that complication rates in this population remain high. Further prospective cohort studies assessing rates of complications within specific pediatric oncology cohorts and by device type are needed so that interventions can be implemented to reduce the morbidity and mortality associated with CVAD dysfunction. Future studies involving RCTs focused on device selection in different pediatric cancer cohorts would be beneficial to enable evidence-based device selection in these patients, particularly to determine the role of PICCs in pediatric cancer care. In addition, we need to hold researchers accountable to the minimum standards of reporting for vascular access studies, in order to improve the quality of research.
Data Availability
Data are available from the corresponding author upon reasonable request.
Abbreviations
- CVAD:
-
Central venous access devices
- CLABSI:
-
Central line–associated bloodstream infection
- VTE:
-
Venous thromboembolism
- TCVC:
-
Tunneled central venous catheters
- PICC:
-
Peripherally inserted central catheters
- TIVAD:
-
Totally implanted venous access devices
- RCT:
-
Randomized control trial
- CI:
-
Confidence interval
- IR:
-
Incidence rate
- RC:
-
Retrospective cohort
- PC:
-
Prospective cohort
- RCC:
-
Retrospective case–control
References
Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F et al (2017) International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol 18(6):719–731. https://doi.org/10.1016/S1470-2045(17)30186-9
Ullman AJ, Bernstein SJ, Brown E, Aiyagari R, Doellman D, Faustino EVS et al (2020) The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics: miniMAGIC. Pediatrics 145(Suppl 3):S269–S284. https://doi.org/10.1542/peds.2019-3474I
Ullman AJ, Kleidon T, Cooke M, Rickard CM (2017) Substantial harm associated with failure of chronic paediatric central venous access devices. BMJ Case Rep 2017:bcr2016218757. https://doi.org/10.1136/bcr-2016-218757
Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM (2015) Complications of central venous access devices: a systematic review. Pediatrics 136(5):e1331–e1344. https://doi.org/10.1542/peds.2015-1507
van den Bosch CH, van der Bruggen JT, Frakking FNJ, Terwisscha van Scheltinga CEJ, van de Ven CP, van Grotel M, et al. Incidence, severity and outcome of central line related complications in pediatric oncology patients; a single center study. J Pediatr Surg. 2019;54(9):1894–900. https://doi.org/10.1016/j.jpedsurg.2018.10.054.
Hord JD, Lawlor J, Werner E, Billett AL, Bundy DG, Winkle C et al (2016) Central line associated blood stream infections in pediatric hematology/oncology patients with different types of central lines. Pediatr Blood Cancer 63(9):1603–1607. https://doi.org/10.1002/pbc.26053
Athale UH, Siciliano S, Cheng J, Thabane L, Chan AK (2012) Central venous line dysfunction is an independent predictor of poor survival in children with cancer. J Pediatr Hematol Oncol 34(3):188–193. https://doi.org/10.1097/MPH.0b013e31823dd284
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Brooke BS, Schwartz TA, Pawlik TM (2021) MOOSE reporting guidelines for meta-analyses of observational studies. JAMA Surg 156(8):787–788. https://doi.org/10.1001/jamasurg.2021.0522
O’Grady NP, Kadri SS (2018) Central venous catheter failures: nowhere near zero. Crit Care Med 46(12):2054–2056. https://doi.org/10.1097/ccm.0000000000003479
(NHSN) (2024) NHSN: bloodstream infection event (central line-associated bloodstream infection and non-central line associated bloodstream infection. https://www.cdc.gov/nhsn/PDFs/pscManual/4PSC_CLABScurrent.pdf. Accessed 10/01/2024
Wright MO, Decker SG, Allen-Bridson K, Hebden JN, Leaptrot D (2018) Healthcare-associated infections studies project: an American Journal of Infection Control and National Healthcare Safety Network data quality collaboration: location mapping. Am J Infect Control 46(5):577–578. https://doi.org/10.1016/j.ajic.2017.12.012
Fratino G, Molinari AC, Parodi S, Longo S, Saracco P, Castagnola E et al (2005) Central venous catheter-related complications in children with oncological/hematological diseases: an observational study of 418 devices. Ann Oncol 16(4):648–654. https://doi.org/10.1093/annonc/mdi111
Barnacle A, Arthurs OJ, Roebuck D, Hiorns MP (2008) Malfunctioning central venous catheters in children: a diagnostic approach. Pediatr Radiol. 38(4):363–78. https://doi.org/10.1007/s00247-007-0610-2. (quiz 486-7)
Tabatabaie O, Kasumova GG, Eskander MF, Critchlow JF, Tawa NE, Tseng JF (2017) Totally implantable venous access devices: a review of complications and management strategies. Am J Clin Oncol 40(1):94–105. https://doi.org/10.1097/coc.0000000000000361
Innovation VH. Covidence systematic review software. Melbourne, Australia. p. www.covidence.org. Accessed 15 Apr 2023
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Freeman MF, Tukey JW (1950) Transformations related to the angular and the square root. Ann Math Stat 21(4):607–611
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36(3):1–48. https://doi.org/10.18637/jss.v036.i03
IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ (2016) Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 6(7):e010247. https://doi.org/10.1136/bmjopen-2015-010247
Deeks J, Higgins JPT, Altman DG (ed) (2022) Chapter 10: Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions: Version 6.4 (update August 2023). Cochrane, 2023. Available from: https://www.training.cochrane.org/handbook
Team RC (2022) R: a language and environment for statistical computing. Austria, Vienna
Hong QN, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P et al (2018) The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ Inf 34:285–291. https://doi.org/10.3233/EFI-180221
Martynov I, Raedecke J, Klima-Frysch J, Kluwe W, Schoenberger J. (2018) Outcome of landmark-guided percutaneously inserted tunneled central venous catheters in infants and children under 3 years with cancer. Pediatr Blood and Cancer. 65(10). https://doi.org/10.1002/pbc.27295.
Lücking V, Rosthoj S (2013) Prediction of bacteremia in children with febrile episodes during chemotherapy for acute lymphoblastic leukemia. Pediatr Hematol Oncol 30(2):131–140. https://doi.org/10.3109/08880018.2012.748111
Cesca E, Dall’igna P, Boscolo-Berto R, Meneghini L, Petris MG, Zanon GF et al (2014) Impact of severe neutropenia and other risk factors on early removal of implanted central venous catheter (ICVC) in children with hematologic malignancies. J Pediatr Hematol Oncol 36(7):541. https://doi.org/10.1097/MPH.0000000000000158
Zachariah M, Al-Yazidi L, Bashir W, Al Rawas AH, Wali Y, Pathare AV (2014) Spectrum of external catheter-related infections in children with acute leukemia-single-center experience. J Infect Public Health 7(1):38–43. https://doi.org/10.1016/j.jiph.2013.06.005
Onyeama SJN, Hanson SJ, Dasgupta M, Baker K, Simpson PM, Punzalan RC (2018) Central venous catheter-associated venous thromboembolism in children with hematologic malignancy. J Pediatr Hematol Oncol 40(8):e519–e524. https://doi.org/10.1097/MPH.0000000000001229
Gidl A, Füreder A, Benesch M, Dworzak M, Engstler G, Jones N et al (2022) Incidence and risk factors of venous thromboembolism in childhood acute lymphoblastic leukaemia–a population-based analysis of the Austrian Berlin-Frankfurt-Münster (BFM) study group. Pediatr Hematol Oncol. https://doi.org/10.1080/08880018.2022.2089791
Gonzalez G, Davidoff AM, Howard SC, Pui CH, Rao BN, Shenep JL et al (2012) Safety of central venous catheter placement at diagnosis of acute lymphoblastic leukemia in children. Pediatr Blood Cancer 58(4):498–502. https://doi.org/10.1002/pbc.24010
Ruiz-Llobet A, Gassiot S, Sarrate E, Zubicaray J, Dapena JL, Rives S et al (2022) Venous thromboembolism in pediatric patients with acute lymphoblastic leukemia under chemotherapy treatment. Risk factors and usefulness of thromboprophylaxis. Results of LAL-SEHOP-PETHEMA-2013. J Thromb Haemost 20(6):1390–9. https://doi.org/10.1111/jth.15699
Jarvis KB, Tveiterås M, de Lange C, Ruud E (2019) Central venous line-related thromboembolism is common in children with non-high-risk acute lymphoblastic leukaemia. Acta Paediatr, Int J Paediatr 108(6):1167–1168. https://doi.org/10.1111/apa.14757
Noailly Charny PA, Bleyzac N, Ohannessian R, Aubert E, Bertrand Y, Renard C (2018) Increased risk of thrombosis associated with peripherally inserted central catheters compared with conventional central venous catheters in children with leukemia. J Pediatr 198:46–52. https://doi.org/10.1016/j.jpeds.2018.03.026
Fu AB, Hodgman EI, Burkhalter LS, Renkes R, Slone T, Alder AC (2016) Long-term central venous access in a pediatric leukemia population. J Surg Res 205(2):419–425. https://doi.org/10.1016/j.jss.2016.06.052
van den Bosch CH, Spijkerman J, Wijnen MHWA, Hovinga ICLK, Meyer-Wentrup FAG, van der Steeg AFW et al (2022) Central venous catheter–associated complications in pediatric patients diagnosed with Hodgkin lymphoma: implications for catheter choice. Support Care Cancer 30(10):8069–8079. https://doi.org/10.1007/s00520-022-07256-3
White AD, Othman D, Dawrant MJ, Sohrabi S, Young AL, Squire R (2012) Implantable versus cuffed external central venous catheters for the management of children and adolescents with acute lymphoblastic leukaemia. Pediatr Surg Int 28(12):1195–1199. https://doi.org/10.1007/s00383-012-3213-4
Abate ME, Sánchez OE, Boschi R, Raspanti C, Loro L, Affinito D et al (2014) Analysis of risk factors for central venous catheter-related complications: a prospective observational study in pediatric patients with bone sarcomas. Cancer Nurs 37(4):292–298. https://doi.org/10.1097/NCC.0b013e31829627e7
Bratton J, Johnstone PAS, McMullen KP (2014) Outpatient management of vascular access devices in children receiving radiotherapy: complications and morbidity. Pediatr Blood Cancer 61(3):499–501. https://doi.org/10.1002/pbc.24642
Moell J, Svenningsson A, Af Sandeberg M, Larsson M, Heyman M, Harila-Saari A et al (2019) Early central line-associated blood stream infections in children with cancer pose a risk for premature catheter removal. Acta Paediatr, Int J Paediatr 108(2):361–366. https://doi.org/10.1111/apa.14432
Park M, Seo Y-m, Shin YJ, Han JW, Cho E, Jang H (2021) Factors affecting the timing of a central line associated bloodstream infection onset in children with cancer. J Pediatr Oncol Nursing. 38(1):26–35. https://doi.org/10.1177/1043454220966831
Schoot RA, de Wetering MD, Stijnen T, Tissing WJE, Michiels E, Abbink FCH et al (2016) Prevalence of symptomatic and asymptomatic thrombosis in pediatric oncology patients with tunneled central venous catheters. Pediatr Blood Cancer 63(8):1438–1444. https://doi.org/10.1002/pbc.26036
Albisetti M, Kellenberger CJ, Bergsträsser E, Niggli F, Kroiss S, Rizzi M et al (2013) Port-a-cath-related thrombosis and postthrombotic syndrome in pediatric oncology patients. J Pediatr 163(5):1340–1346. https://doi.org/10.1016/j.jpeds.2013.06.076
Redkar R, Bangar A, Krishnan J, Raj V, Swathi C, Joshi S (2019) Role of chemoports in children with hematological/solid tumor malignancies - technical implications and complications: An institutional experience. J Indian Assoc Pediatr Surg 24(1):27–30. https://doi.org/10.4103/jiaps.JIAPS_212_17
Rykov MY, Zaborovskij SV, Shvecov AN, Shukin VV (2018) Peripherally inserted central catheters in the treatment of children with cancer: results of a multicenter study. J Vascular Access 19(4):378–381. https://doi.org/10.1177/1129729818757970
Ullman AJ, Condon P, Edwards R, Gibson V, Takashima M, Schults J et al (2020) Prevention of occlusion of cEnTral lInes for children with cancer: an implementation study. J Paediatr Child Health 56(12):1875–1884. https://doi.org/10.1111/jpc.15067
Viana Taveira MR, Lima LS, Araújo CC, Mello MJG, de Araújo CC, de Mello MJG (2017) Risk factors for central line-associated bloodstream infection in pediatric oncology patients with a totally implantable venous access port: a cohort study. Pediatr Blood Cancer 64(2):336–342. https://doi.org/10.1002/pbc.26225
Mangum DS, Verma A, Weng C, Sheng X, Larsen R, Kirchhoff AC et al (2013) A comparison of central lines in pediatric oncology patients: early removal and patient centered outcomes. Pediatr Blood Cancer 60(11):1890–1895. https://doi.org/10.1002/pbc.24687
Khera S, Kumar A, Parikh B, Simalti AK, Davera S, Mahajan P, et al (2023) Safety and outcome of ultrasound-guided tunneled central venous catheter in children with cancers from low middle-income country: a prospective study. Pediatric Blood and Cancer. 70(1). https://doi.org/10.1002/pbc.30029.
Miliaraki M, Katzilakis N, Chranioti I, Stratigaki M, Koutsaki M, Psarrou M et al (2017) Central line-associated bloodstream infection in childhood malignancy: single-center experience. Pediatr Int 59(7):769–775. https://doi.org/10.1111/ped.13289
Gowin E, Świątek-Kościelna B, Mańkowski P, Januszkiewicz-Lewandowska D (2020) The profile of microorganisms responsible for port-related bacteremia in pediatric hemato-oncological patients. Cancer Control 27(1):1073274820904696. https://doi.org/10.1177/1073274820904696
Cher WQ, Lee V, Wang R, Cheah SM, Lee YT, Saffari SE et al (2022) Postoperative rather than preoperative neutropenia is associated with early catheter-related bloodstream infections in newly diagnosed pediatric cancer patients. Pediatr Infect Dis J 41(2):133–139. https://doi.org/10.1097/inf.0000000000003315
Beck O, Muensterer O, Hofmann S, Rossmann H, Poplawski A, Faber J, et al (2019) Central venous access devices (CVAD) in pediatric oncology patients—a single-center retrospective study over more than 9 years. Frontiers in Pediatrics 7(JUN). https://doi.org/10.3389/fped.2019.00260.
Buonpane CL, Lautz TB, Langer M (2022) High rates of central venous line replacement or revision in children with cancer at US children’s hospitals. J Pediatr Hematol Oncol 44(2):43–46. https://doi.org/10.1097/MPH.0000000000002098
Schoot RA, Van Ommen CH, Stijnen T, Tissing WJE, Michiels E, Abbink FCH et al (2015) Prevention of central venous catheter-associated bloodstream infections in paediatric oncology patients using 70% ethanol locks: a randomised controlled multi-centre trial. Eur J Cancer 51(14):2031–2038. https://doi.org/10.1016/j.ejca.2015.06.126
Berrueco R, Rives S, Catala A, Toll T, Gene A, Ruiz A et al (2013) Prospective surveillance study of blood stream infections associated with central venous access devices (port-type) in children with acute leukemia: an intervention program. J Pediatr Hematol Oncol 35(5):e194–e199. https://doi.org/10.1097/MPH.0b013e318290c24f
Celebi S, Sezgin ME, Cakir D, Baytan B, Demirkaya M, Sevinir B et al (2013) Catheter-associated bloodstream infections in pediatric hematology-oncology patients. Pediatr Hematol Oncol 30(3):187–194. https://doi.org/10.3109/08880018.2013.772683
Wiegering V, Schmid S, Andres O, Wirth C, Wiegering A, Meyer T, et al (2014) Thrombosis as a complication of central venous access in pediatric patients with malignancies: a 5-year single-center experience. BMC Hematology 14(1). https://doi.org/10.1186/2052-1839-14-18.
Zakhour R, Hachem R, Alawami HM, Jiang Y, Michael M, Chaftari A-M et al (2017) Comparing catheter-related bloodstream infections in pediatric and adult cancer patients. Pediatr Blood Cancer 64(10):e26537. https://doi.org/10.1002/pbc.26537
Kristinsdottir I, Haraldsson A, Gudlaugsson O, Thors V (2021) Low risk of central line-associated bloodstream infections in pediatric hematology/oncology patients. Pediatric Infectious Disease Journal 40(9):827–831. https://doi.org/10.1097/INF.0000000000003177
Mokone L, Ndove P, Magooa K, Tsilo K, Rampeta R, Brits E et al (2021) Complications associated with central venous lines for paediatric oncology patients at Universitas Academic Hospital, Bloemfontein, from 1992 to 2018. South African Journal of Child Health 15(4):189–192. https://doi.org/10.7196/SAJCH.2021.v15i4.1778
Martynov I, Schoenberger J (2021) Impact of perioperative absolute neutrophil count on central line-associated bloodstream infection in children with acute lymphoblastic and myeloid leukemia. Frontiers in Oncology 11 https://doi.org/10.3389/fonc.2021.770698.
Rogers AE, Eisenman KM, Dolan SA, Belderson KM, Zauche JR, Tong S, et al (2017) Risk factors for bacteremia and central line-associated blood stream infections in children with acute myelogenous leukemia: a single-institution report. Pediatr Blood Cancer 64(3). https://doi.org/10.1002/pbc.26254.
Van Den Broek A, Williams VK, Revesz T, Suppiah R (2015) Pediatric thrombotic events: a single-center study. J Pediatr Hematol Oncol 37(4):e210–e214. https://doi.org/10.1097/MPH.0000000000000302
Cho HK (2021) Catheter care bundle and feedback to prevent central line-associated bloodstream infections in pediatric patients. Clin Exp Pediatr 64(3):119–120. https://doi.org/10.3345/cep.2020.01186
Schults J, Kleidon T, Chopra V, Cooke M, Paterson R, Ullman AJ et al (2021) International recommendations for a vascular access minimum dataset: a Delphi consensus-building study. BMJ Qual Saf 30(9):722. https://doi.org/10.1136/bmjqs-2020-011274
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
CRediT Author Statement: Jenna Nunn: Conceptualization, methodology, software, validation, formal analysis, investigation, writing – original draft, review & editing. Mari Takashima: methodology, validation, formal analysis, visualization, writing- review & editing. Erin Wray-Jones: validation, methodology, investigation, writing – review & editing. Trisha Soosay Raj: conceptualization, methodology, writing – review & editing, supervision. Diane Hanna: conceptualization, writing – review & editing, supervision. Amanda Ullman: conceptualization, methodology, writing – review & editing, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nunn, J.L., Takashima, M.D., Wray-Jones, E.M. et al. Central venous access device adverse events in pediatric patients with cancer: a systematic review and meta-analysis. Support Care Cancer 32, 662 (2024). https://doi.org/10.1007/s00520-024-08853-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08853-0