Abstract
Background
Detection of precachexia is important for the prevention and treatment of cachexia. However, how to identify precachexia is still a challenge.
Objective
This study aimed to detect cancer precachexia using a simple method and distinguish the different characteristics of precachexia and cachexia.
Methods
We included 3896 participants in this study. We used all baseline characteristics as input variables and trained machine learning (ML) models to calculate the importance of the variables. After filtering the variables based on their importance, the models were retrained. The best model was selected based on the receiver operating characteristic value. Subsequently, we used the same method and process to identify patients with precachexia in a noncachexia population using the same method and process.
Results
Participants in this study included 2228 men (57.2%) and 1668 women (42.8%), of whom 471 were diagnosed with precachexia, 1178 with cachexia, and the remainder with noncachexia. The most important characteristics of cachexia were eating changes, arm circumference, high-density lipoprotein (HDL) level, and C-reactive protein albumin ratio (CAR). The most important features distinguishing precachexia were eating changes, serum creatinine, HDL, handgrip strength, and CAR. The two logistic regression models for screening for cachexia and diagnosing precachexia had the highest area under the curve values of 0.830 and 0.701, respectively. Calibration and decision curves showed that the models had good accuracy.
Conclusion
We developed two models for identifying precachexia and cachexia, which will help clinicians detect and diagnose precachexia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer cachexia is a multifactorial wasting syndrome characterized by involuntary weight loss [1]. It significantly impacts the quality of life of cancer patients and exacerbates chemotherapy toxicity, thereby substantially increasing cancer mortality [2]. Cachexia affects 50% of cancer patients and accounts for 20% of cancer-related deaths [3, 4].
It is now widely recognized that cachexia responds poorly to available nutritional and pharmacological treatments and eventually and inevitably leads to patient death once a critical point is reached [5]. Prevention and early recognition of cachexia deserve more attention than the treatment of cachexia because once the patient enters cachexia, he or she enters a disease process that is almost irreversible [6]. Owing to the complex presentation of cancer cachexia and its specificity in different patients, there is no precise definition or diagnosis of cachexia, and the current diagnostic criteria rely on the international expert consensus on cachexia reached in 2011. This consensus describes cancer cachexia as a continuum of three stages: precachexia, cachexia, and refractory cachexia [7]. However, although this consensus provides a framework for the diagnosis of cachexia, in practice, precachectic presentation is very insidious and difficult for clinicians to detect. The lack of pathophysiological evidence for cachexia has led clinicians to rely on their own experience or research strength to describe cachexia [8]. Patients also sometimes ignore weight changes, making the identification of cachexia more difficult in the absence of information on weight loss. Therefore, there is a need to develop other methods to identify cachexia and its stages.
Typically, patients with cancer undergo extensive physical examinations and inquiries from medical staff during their hospital stay. The results of these examinations and the patient’s self-reported physical condition provide a great deal of information that may be beneficial in identifying cachexia at an early stage. However, their potential value is underutilized. Much of the current research on cachexia has focused on the relationship between biomarkers and prognosis; however, little attention has been paid to precachexia studies. Machine learning is becoming increasingly popular because of its suitability for handling biological data of an ever-increasing size and for analyzing the complexity inherent in diseases [9]. In medical research, machine learning can be applied to clinical datasets to develop powerful risk models to reclassify patients [10]. Therefore, we aimed to use machine learning methods, combined with hematological information and symptoms of cancer patients, to identify individuals in precachexia and cachexia.
Methods
Study design and population
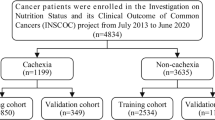
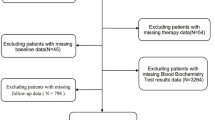
The Investigation on Nutrition Status and Clinical Outcome of Common Cancers (INSCOC) was a multi-center cancer cohort study in China. Data of this study were collected between 2013 and 2019 [11]. This study included patient with cancer older than 20 years and hospitalized ≥ 48 h. Figure 1 shows the specific inclusion and exclusion process. In the end, 3896 participants were included in the study.
The definition of cachexia was from the 2011 International Consensus on Cancer Cachexia [7]. A cachexia participant was considered cachectic if she had lost more than 5% of her body weight involuntarily in the last 6 months, or more than 2% with a combined BMI < 20 kg/m2. Simple weight loss of more than 2% but not more than 5% was considered to be pre-cachexia. Participants were distinguished as non-cachexia (n = 2247), pre-cachexia (n = 471), and cachexia (n = 1178).
In accordance with the principles of the Declaration of Helsinki, this study was approved by the Medical Ethical Review Committee of the hospital where it was conducted (registration number: ChiCTR1800020329). Each participant provided informed consent. All participants’ information was obtained after participants signed informed consent.
Variables
The basic patient characteristics collected in this study include demographics, laboratory test results, physical measurements, and physical symptoms. All personal patient information was deidentified. Demographic data included sex, age, and basic disease information such as tumor type and stage. The tumor stage was recorded as the stage of the patient at first diagnosis.
All laboratory tests were conducted within 48 h of hospitalization after the patients had fasted for at least 9 h. Blood tests included blood lipid, protein, creatinine, glucose, and transaminase levels, and neutrophil, white blood cell, platelet, and lymphocyte counts. The composite indicators were calculated on this basis. Platelet-lymphocyte ratio: platelet count (× 109)/lymphocyte count (× 109); neutrophil lymphocyte ratio: neutrophil count (× 109)/lymphocyte count (× 109); leukocyte lymphocyte ratio: white blood cell count (× 109)/lymphocyte count (× 109); prognostic nutritional index: albumin (g/L) + 5 × lymphocyte count (× 109); C-reactive protein-albumin ratio (CAR): C-reactive protein (mg/L)/albumin (g/L); triglycerides-glucose index: ln (triglycerides (mg/dL) × glucose (mg/dL)/2).
Physical measurements included arm circumference (MAC), calf circumference (CAC), triceps skinfold thickness (TSF), and hand grip strength (HGS). HGS was measured using an electronic handheld dynamometer (EH101; CAMRY, Guangdong, China). The patients were instructed to stand comfortably and perform three maximal isometric contractions with the nondominant hand 30 s apart. For post-mastectomy patients, measurements should be taken using the side not subjected to lymph node dissection.
Eating changes, gastrointestinal symptoms, and decreased physical activity were reported by the participants, and fever, ankle edema, and ascites were assessed by health care providers.
Machine learning models
Our study was divided into two parts: the first distinguishing between patients with noncachexia and cachexia and the second distinguishing between patients with precachexia and noncachexia. Before building the machine learning models, the two groups of participants were divided into training and validation sets at a ratio of 7:3. Precachexia and cachexia were defined as dichotomous outcome variables.
We first developed five models to calculate variable importance ranking, namely, logistic regression (LR), least absolute selection and shrinkage operator regression (LASSO), eXtreme gradient boosting (XGB), random forest (RF), and decision tree (DT). To improve the performance of the models, we used tenfold cross-validation when training the models and hyperparameter tuning in the RF and DT models.
According to the ranking chart of variable importance, we determined the importance of each variable according to the number of occurrences and filtered out variables with more than four occurrences. To avoid model overfitting, we calculated the correlation between the variables, excluded those with high correlation, and then incorporated the remaining variables into the models for the second model training. We used receiver operating characteristic (ROC) curves to calculate the area under the curve (AUC) values of the models in the validation set to compare the judgment performances of the different models. After determining the optimal model, a decision curve was used to test its accuracy and a nomogram was established.
Statistical analysis
For statistical data, we used the mean ± standard deviation for normally distributed continuous variables, and the median [interquartile range] for continuous variables that did not follow a normal distribution. The normality of the variables was assessed using the Shapiro–Wilk test. The Student’s t-test or Kruskal–Wallis test was used for continuous variables, and the chi-square test was used for categorical variables.
All tests were two-sided, and statistical significance was set at P < 0.05. All analyses were performed using statistical software (R Studio version 4.2.0).
Result
Characteristics of the participants
The study included 3896 participants, including 2247 patients with noncachexia, 471 with precachexia, and 1178 patients with cachexia. The mean age of all participants was 58.49 ± 11.10 years, including 2228 men (57.2%) and 1668 women (42.8%). Among the participants diagnosed with precachexia, 61.4% were men; among the participants diagnosed with cachexia, 62.7% were men (Table 1). The tumor types in all participants included lung, stomach, liver, colorectal, breast, esophageal, cervical, endometrial, nasopharyngeal, pancreatic, ovarian, prostate, bladder, cholangiocarcinoma, and other cancers. The number and percentage of different cancer types are presented in Table 2. The results indicate that lung cancer is the most common, accounting for 34.8% of the cases. Among all cancer types, the highest incidences of cachexia were observed in pancreatic cancer, gastric cancer, and cholangiocarcinoma, with pancreatic cancer having the highest cachexia incidence rate at 48.15% (Table 2).
Variable screening and model training
First, we performed model training to filter out appropriate variables. We used all baseline features, except weight change, as input variables in the training set for model training and optimization. The training and validation sets were divided at a 7:3 ratio (Supplementary Tables S1 and S2). The variable importance was calculated and ranked separately for all models. The top ten variables in importance are shown in Supplementary Figure S1 and S2. Based on the variable importance ranking chart, we listed the number of occurrences of each variable in all the models and ranked the variable importance again according to the number of occurrences (Table 3; Table 4). We selected variables with greater than four occurrences, from which we know that the most important features in identifying cachexia were eating changes, MAC, high-density lipoprotein (HDL), and CAR. The most important features for identifying precachexia were eating changes, serum creatinine (Scr), HDL, HGS, and CAR. Subsequently, we performed correlation tests between the variables, and the results showed a high correlation between MAC and CAC (r = 0.64). Since MAC was more important than CAC in Table 2, therefore, we excluded MAC (Supplementary Figure S3).
Filtered variables were used to train the models separately to identify cachexia and precachexia. The results showed that the LR models had the best recognition effect for both cachexia and precachexia, with AUC values of 0.830 and 0.701, respectively, for the ROC curves (Figs. 2 and 3).
ROC curves to differentiate between cachexia and non-cachexia (including pre-cachexia). a First model training to filter variables. b Second model training to screen the best model LR, logistic regression model; LASSO, least absolute selection and shrinkage operator regression model; XGB, eXtreme gradient boosting; RF, random forest; DT, decision tree
ROC curves to differentiate between pre-cachexia and non-cachexia. a First model training to filter variables. b Second model training to screen the best model. LR, logistic regression model; LASSO, least absolute selection and shrinkage operator regression model; XGB, eXtreme gradient boosting; RF, random forest; DT, decision tree
Model validation and development of nomograms
There were significant associations between variables in the model and precachexia or cachexia (Supplementary Tables S3). To assess the accuracy of the models, we plotted decision and calibration curves for the established LR models. The results indicated that the LR models performed well in identifying noncachexia from precachexia and cachexia (Fig. 4). Therefore, we used the LR models to create separate nomograms to identify precachexia and cachexia (Fig. 5).
Discussion
In this large retrospective study of 3896 patients with cancer, we used machine learning methods to identify patients with precachexia and cachexia. After screening for these characteristics, we constructed a screening model for cachexia risk and a diagnostic model for identifying patients with precachexia. These models have good identification efficacy, and nomograms were created based on these models. This may help clinicians detect precachexia and cachexia in a timely manner and improve the outcomes of cancer patients.
Despite the high prevalence of cachexia in cancer patients, evidence-based interventions remain scarce, and a clear definition of cachexia and effective screening tools are urgently needed [12]. A previous study used the machine learning method to identify cachexia without weight loss information [13]. However, their study did not identify precachexia. The identification of precachexia is crucial, as none of the current treatments can reverse it effectively, and refractory cachexia does not respond to treatment [14]. Regardless of its prevalence, a systematic search for precachexia is a powerful tool to prevent the onset of venting or delay the devastating picture of cancer cachexia [15]. Therefore, we wanted to develop an effective method to identify precachexia to fill the gap in this area.
In this study, we screened a large amount of clinical information to identify precachexia and cachexia in an easy and economical manner. We found that cachectic patients were characterized by eating changes, MAC, HDL, and CAR, whereas precachectic patients were characterized by eating changes, Scr, HDL, HGS, and CAR. Of these, eating changes, HDL, and CAR were common to both, suggesting that these features persisted in the cachectic antecedent.
Scr and HGS are unique features of precachexia, and both are associated with muscle status. Under normal renal function, creatinine is usually produced in the body at a relatively constant rate depending on the absolute muscle mass [16]. Previous studies have demonstrated a significant correlation between creatinine levels and muscle mass. Previous studies estimating muscle mass based on creatinine kinetics showed good correlation between creatinine and other indicators of muscle mass [17]. Scr levels are highly correlated with lean body mass [18]. Increased creatinine levels may result from increased muscle catabolism. A prospective study suggested that creatinine might be a surrogate indicator for assessing sarcopenia in advanced lung cancer [19]. Our results showed that muscle loss manifests differently at different stages of cachexia. Precachexia is manifested by elevated Scr and decreased HGS, whereas cachexia is manifested by decreased absolute muscle mass. A previous study on precachetic lung cancer showed unchanged muscle mass but significantly reduced muscle function in the precachetic stage. Our findings are consistent with them, showing only a decrease in HGS but not in muscle mass in the precachexia phase.
In addition, low HGS is not only associated with cachexia survival but also has a synergistic effect with inflammation [20]. A previous study showed that muscular inflammatory signaling and UPS activity were not altered in patients with precachexia [21]. It transitions from systemic to local inflammation to initiate cachexia, which is a characteristic of sarcopenia. There is a synergistic effect between inflammation and sarcopenia that promotes each other and mediates the progression from precachexia to cachexia. Cachexia is an active catabolic process whose high catabolism is mainly attributed to the systemic inflammatory response caused by the tumor itself, which promotes the catabolism of fats and proteins, and this inflammatory response persists [22, 23]. Our study shows that CAR is common to both precachexia and cachexia, suggesting that inflammation persists during the development of cachexia, which is consistent with the findings of previous study. Previous studies have suggested that systemic inflammation is significantly associated with cachexia prognosis [24]. The etiology of cachexia is not fully understood, but the vast majority of patients appears to have chronic systemic inflammation [25]. Therefore, we should pay sufficient attention to the persistent inflammatory state, which may predict the development of sarcopenia and cachexia and is associated with a poorer prognosis.
In addition to sarcopenia and inflammatory, changes in eating are another characteristic of cachexia. Changes in eating is the most important features of cachexia and precachexia. Reportedly, reduced food intake was strongly associated with weight loss [26]. An international multicenter cohort analysis suggested that reduced eating predicted a high probability of weight loss and was associated with poor prognosis [27]. Reduced eating in patients with cancer is multifactorial and includes tumor growth, protein and fat hydrolysis, cytokine release, systemic inflammation, intestinal obstruction, and response to chemotherapy [28]. Tumor-released substances, such as pro-inflammatory cytokines, lactate, and parathyroid hormone releases peptides (PTHRP), are decisive factors in diagnosing anorexia nervosa [29]. Anorexia and weight loss are two major features of cancer cachexia that are regulated by two independent mechanisms, and anorexia occurs earlier than wasting in the course of cachexia [30]. Increased tumor and resting energy expenditure are important causes of progressive cachexia in cancer patients with anorexia, which further alters the metabolic and inflammatory responses of cancer patients [28]. Although a decline in eating cannot be fully equated with anorexia, it should be taken seriously when a patient with cancer shows this symptom, as this may indicate that the patient is entering the elusive precachexia phase. Decreased eating is present throughout the course of cachexia and seems to be a warning sign for this disease.
Our study innovatively developed a diagnostic model for precachexia. Although the study is preliminary, it is informative for the clinical diagnosis of precachexia. In the present study, precachexia and cachexia were screened separately from noncachexia for characteristics, and in future studies, a diagnostic model with three outcome variables could be further developed to identify noncachexia, precachexia and cachexia. In addition, precachetic and cachetic sarcopenia may be different, and the molecular mechanisms involved deserve further investigation. Sarcopenia was originally defined by the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) in the geriatric population as a progressive systemic skeletal muscle disease that involves reduction of muscle mass and loss of muscle function, mainly represented by muscle strength [31, 32]. Treatment of sarcopenia in precachexia may be a potential treatment modality to prevent disease progression.
This is the first study to use machine learning methods to identify precachexia. The data and results are generalizable to participants from multiple large hospitals across the country, including those with multiple cancer types. However, this study still has several limitations. First, our definitions of cachexia and precachexia come from the 2011 International Expert Consensus, which relies on the patient’s recollection of his or her weight changes over the past 6 months and may lead to errors. The latest guidelines on cachexia suggest that changes in muscle mass should also be taken into account [33]. However, due to data limitations, we were unable to further explore the role of modeling in predicting muscle loss in this study. Second, our distinction between the cachectic stages should include the cachectic refractory stage; however, machine learning was not supported because of numerical limitations. Similarly, owing to population limitations, we did not apply the models to different tumor types, but we should explore whether the models we developed are suitable for all tumor types in future studies. Furthermore, in future studies, we should validate the model we developed for identifying precachexia patients in larger cancer cohorts.
Conclusion
Our study established two LR models to identify precachexia and cachexia and demonstrated the same and specific characteristics of the different cachexia stages. The variables used in the model are part of routine inpatient examinations, which are easily accessible and do not add an additional burden to cancer patients. This may inform and assist clinicians in identifying and diagnosing early stage versus full cachexia and help guide management strategies to optimize outcomes in cancer patients.
Data availability
No datasets were generated or analysed during the current study.
References
Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH (2018) Cancer-associated cachexia. Nat Rev Dis Primers 4:17105
Fearon K, Arends J, Baracos V (2013) Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 10(2):90–99
Argilés JM, Busquets S, Stemmler B, López-Soriano FJ (2014) Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 14(11):754–762
Li X, Hu C, Zhang Q, Wang K, Li W, Xu H, et al. (2022) Cancer cachexia statistics in China. Precis Nutr 1(1). https://doi.org/10.1097/PN9.e00008
Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G et al (2010) Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics.” Clin Nutr 29(2):154–159
Cui J, Liu Y, Li F, Zhuo W, Huang L, Xu C et al (2023) Evidence-based guideline on immunonutrition in patients with cancer. Precis Nutr 2(1):e00031
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12(5):489–495
Roeland EJ (2022) Cancer cachexia: the elephant in the room? J Cachexia Sarcopenia Muscle 13(1):3–4
Greener JG, Kandathil SM, Moffat L, Jones DT (2022) A guide to machine learning for biologists. Nat Rev Mol Cell Biol 23(1):40–55
Deo RC (2015) Machine learning in medicine. Circulation 132(20):1920–1930
Xu H, Song C, Yin L, Wang C, Fu Z, Guo Z et al (2022) Extension protocol for the Investigation on Nutrition Status and Clinical Outcome of Patients with Common Cancers in China (INSCOC) study: 2021 update. Precis Nutr 1(2):e00014
Baracos VE, Coats AJ, Anker SD, Sherman L, Klompenhouwer T (2022) Identification and management of cancer cachexia in patients: assessment of healthcare providers’ knowledge and practice gaps. J Cachexia Sarcopenia Muscle 13(6):2683–2696
Yin L, Cui J, Lin X, Li N, Fan Y, Zhang L et al (2022) Identifying cancer cachexia in patients without weight loss information: machine learning approaches to address a real-world challenge. Am J Clin Nutr 116:1229
O’Connell TM, Golzarri-Arroyo L, Pin F, Barreto R, Dickinson SL, Couch ME et al (2021) Metabolic biomarkers for the early detection of cancer cachexia. Front Cell Dev Biol 9:720096
Muscaritoli M, Aversa Z, Lucia S (2014) The conundrum of pre-cachexia existence. Clin Nutr 33(6):1160
Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S (1983) Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr 37(3):478–494
Delgado C, Johansen KL (2020) Revisiting serum creatinine as an indicator of muscle mass and a predictor of mortality among patients on hemodialysis. Nephrol Dial Transplant 35(12):2033–2035
Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM et al (2008) Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol 3(2):348–354
Tang T, Xie L, Hu S, Tan L, Lei X, Luo X et al (2022) Serum creatinine and cystatin C-based diagnostic indices for sarcopenia in advanced non-small cell lung cancer. J Cachexia Sarcopenia Muscle 13(3):1800–1810
Song M, Zhang Q, Tang M, Zhang X, Ruan G, Zhang X et al (2021) Associations of low hand grip strength with 1 year mortality of cancer cachexia: a multicentre observational study. J Cachexia Sarcopenia Muscle 12(6):1489–1500
Op den Kamp CM, Langen RC, Minnaard R, Kelders MC, Snepvangers FJ, Hesselink MK et al (2012) Pre-cachexia in patients with stages I-III non-small cell lung cancer: systemic inflammation and functional impairment without activation of skeletal muscle ubiquitin proteasome system. Lung Cancer 76(1):112–7
Narasimhan A, Shahda S, Kays JK, Perkins SM, Cheng L, Schloss KNH et al (2020) Identification of potential serum protein biomarkers and pathways for pancreatic cancer cachexia using an aptamer-based discovery platform. Cancers (Basel) 12(12):3787
Poulia KA, Sarantis P, Antoniadou D, Koustas E, Papadimitropoulou A, Papavassiliou AG et al (2020) Pancreatic cancer and cachexia-metabolic mechanisms and novel insights. Nutrients 12(6):1543
Zhang Q, Song M-M, Zhang X, Ding J-S, Ruan G-T, Zhang X-W et al (2021) Association of systemic inflammation with survival in patients with cancer cachexia: results from a multicentre cohort study. J Cachexia Sarcopenia Muscle 12(6):1466–1476
Seelaender M, Laviano A, Busquets S, Püschel GP, Margaria T, Batista ML (2015) Inflammation in cachexia. Mediators Inflamm 2015:536954
Giacosa A, Frascio F, Sukkar SG, Roncella S (1996) Food intake and body composition in cancer cachexia. Nutrition 12(1 Suppl):S20–S23
Martin L, Muscaritoli M, Bourdel-Marchasson I, Kubrak C, Laird B, Gagnon B et al (2021) Diagnostic criteria for cancer cachexia: reduced food intake and inflammation predict weight loss and survival in an international, multi-cohort analysis. J Cachexia Sarcopenia Muscle 12(5):1189–1202
Archid R, Solass W, Tempfer C, Königsrainer A, Adolph M, Reymond MA et al (2019) Cachexia anorexia syndrome and associated metabolic dysfunction in peritoneal metastasis. Int J Mol Sci 20(21):5444
Peixoto da Silva S, Santos JMO, Costa e Silva MP, da Gil RMC, Medeiros R (2020) Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle 11(3):619–35
Yeom E, Yu K (2022) Understanding the molecular basis of anorexia and tissue wasting in cancer cachexia. Exp Mol Med 54(4):426–432
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48(4):601
Liu C, Liu T, Deng L, Zhang Q, Song M, Shi J et al (2024) Sarcopenic obesity and outcomes for patients with cancer. JAMA Netw Open 7(6):e2417115
Arends J, Strasser F, Gonella S, Solheim TS, Madeddu C, Ravasco P et al (2021) Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines☆. ESMO Open 6(3):100092
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing. We are grateful to all the participants who have been part of the project and to the INSCOC database which has enabled this research.
Funding
This work was supported by the National Key Research and Development Program (grant number 2022YFC2010101) and National Natural Science Foundation of China (32100939 to Z. W.), and Hebei Natural Science Foundation (C2022104004 to Z. W.).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. CY wrote the manuscript. CY, LCA, and ZX analyzed and interpreted the patient data, CY, LCA, ZX, XHL, WZW and SHP made substantial contributions to the conception, design, and intellectual content of the studies. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study followed the Helsinki declaration. All participants signed an informed consent form, and this study was approved by the Institutional Review Board of each hospital. Trial registration: The Investigation on Nutrition Status and Clinical Outcome of Common Cancers (INSCOC) (Chinese Clinical Trial Registry: ChiCTR1800020329, URL of registration: http://www.chictr.org.cn/showprojen.aspx?proj=31813).
Consent for publication
Obtained.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Y., Liu, C., Zheng, X. et al. Machine learning to identify precachexia and cachexia: a multicenter, retrospective cohort study. Support Care Cancer 32, 630 (2024). https://doi.org/10.1007/s00520-024-08833-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08833-4