Abstract
Importance
Patients undergoing cancer treatment experience a multitude of skin, hair, and nail adverse events, prompting them to use non-evidence-based and often restrictive over-the-counter (OTC) recommendations to alleviate their symptoms. Comprehensively assessing evidence-based OTC modalities is crucial to enable cancer patients to comfortably resume their lives post-treatment and integrate clinically sound practices into their self-care routines.
Objective
Perform a systematic review and assessment of evidence-based OTC skin, hair, and nail care recommendations for adult patients undergoing cancer treatment.
Evidence review
PubMed, Cochrane, Embase, and Medline databases were searched in March 2023 to identify English articles addressing OTC skin, hair, and nail care recommendations for adult patients before, during, and after cancer chemotherapy or radiation therapy (RT). Quality was assessed with Oxford Centre for Evidence Based Medicine criteria.
Findings
2192 unique articles were screened, of which 77 met inclusion criteria consisting of 54 randomized controlled trials (RCT), 8 non-randomized controlled cohorts, 1 non-randomized controlled clinical trial, 3 controlled prospective cohorts, 4 prospective cohorts, 2 controlled clinical trials, 1 prospective comparative study, 2 case reports, and 2 case series discussing 9322 patients. An additional article outside of our database search was included for a total of 78 articles. OTC skin care treatments with the best quality of evidence included moisturizing creams. Our review revealed a paucity of evidence-based hair and nail care practices.
Conclusions and relevance
This systematic review serves to highlight the efficacy of diverse OTC skin, hair, and nail care recommendations for adult cancer patients while encouraging further clinical trials to establish evidence-based management guidelines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many patients undergoing cancer therapy experience adverse dermatologic events including radiation dermatitis (RD), alopecia, rashes, hyperpigmentation, hand-foot syndrome (HFS), phototoxicity, and nail dystrophy [1, 2]. These patients often face a multitude of challenges, both physical and emotional, related to the dermatologic effects of their treatments. To help mitigate these issues, patients either independently seek or are encouraged by healthcare providers or peers to follow various skin, hair, and nail care recommendations, many of which are non-evidence based and often restrictive (Table 1). There are frequently no or few citations associated with these recommendations and those that are cited often reference narrative reviews lacking actual evidence from clinical trials. In addition to the questionable validity of these recommendations, the restrictive nature of many can cause undue stress and anxiety for patients.
It is important to investigate the validity of cancer therapy skin, hair, and nail care recommendations to identify and promote evidence-based practices. By prioritizing evidence-based recommendations, clinicians can offer patients interventions that have been rigorously studied and proven effective, promoting their well-being, and optimizing their quality of life throughout their cancer journey. Moreover, evidence-based practices empower clinicians to make informed decisions tailored to individual patient needs. This systematic review aims to summarize current evidence-based recommendations in the literature as they pertain to skin, hair, and nail care management for adult patients before, during, and after cancer therapy, along with a quality of evidence assessment for each supporting study.
Methods
We performed a systematic literature search to identify evidence-based OTC skin, hair, and nail care recommendations for adult patients undergoing cancer treatment. Our systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines [11]. Using the PubMed, Cochrane, Embase, and Medline databases, a search for all peer-reviewed articles was performed with the following search terms: “skin care AND chemotherapy,” “skin care AND radiation,” “skin care AND radiotherapy,” “hair care AND chemotherapy,” “hair care AND radiation,” hair care AND radiotherapy,” “nail care AND chemotherapy,” “nail care AND radiation, “nail care AND radiotherapy.”
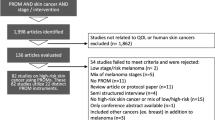
The abstracts were independently screened using defined criteria for eligibility. Inclusion criteria specified that papers be: written in English and discuss studies of OTC interventions addressing skin, hair, and nail changes in adults age 19 or older receiving chemotherapy or RT for cancer. References from included reports were reviewed and additional sources that were not initially identified were added. Articles were excluded if they were review articles, not available in full text, not in English, animal studies, or studies of pediatric patients, or involved prescription-based therapies (Fig. 1). Animal studies were omitted because they might not accurately reflect human physiology, treatment response, or adverse effects, thereby limiting their relevance to clinical decision-making for humans. Pediatric studies were excluded because skin, hair, and nail care practices are much more common in adults and differ between adults and children. This leads to varying OTC recommendations influenced by differing physiology, treatment protocols, and potential adverse effects, thereby limiting their direct applicability to the targeted adult cancer patient population and their providers in this review. Four reviewers (B.J., L.M.P, S.A.R., and E.T.) independently screened all titles and abstracts. Articles that met inclusion criteria underwent full-text review. In case of disagreement, a consensus meeting was held to resolve discrepancies. Quality of evidence was used to evaluate the strength of a particular recommendation and was assessed and classified by the Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence (LoE) as previously described [12]: level 1 (systematic review of RCTs or high-quality randomized controlled trial), level 2 (lesser quality RCT or prospective cohort study), level 3 (case–control study, non-randomized controlled cohort or follow-up study), level 4 (case series), or level 5 (expert opinion, mechanism-based reasoning).
Results
The initial database search provided 2301 total articles with 2192 unique articles after removal of duplicates. Seventy-seven articles met inclusion criteria consisting of 54 RCTs, 8 non-randomized controlled cohorts, 1 non-randomized controlled clinical trial, 3 controlled prospective cohorts, 4 prospective cohorts, 2 controlled clinical trials, 1 prospective comparative study, 2 case reports, and 2 case series discussing 9322 patients. An additional article, an RCT of 22 patients, that met inclusion criteria was added from a reference list of a screened article. A total of 78 studies, including 77 articles from the database search and an outside search article, were included in our final review. OTC skin care treatments with the best quality of evidence included moisturizing creams and lotions. Treatments with moderate quality of evidence and efficacy included antimicrobials and antiseptics, dressings, and natural products. Our review revealed a paucity of evidence-based hair and nail care practices. Included articles and results are summarized in Table 2.
Basic hygiene and routine care
Recommendations pertaining to basic hygiene and routine care exist in other reviews in literature that were not supported by any studies in our systematic review including: avoidance of manicures and pedicures [3], keeping a short hair style, avoiding daily shampoo, avoiding hair manipulation such as using hair clips, dryers, curling irons, dye [10], avoiding shaving the armpit with a straight razor [5], and avoidance of perfume, deodorant, powder, and lotion in the treatment site [6].
We found several evidence-based studies that contraindicate the previous suggestions. In a study, washing the skin with soap and water during the course of treatment was not associated with increased skin toxicity [13]. A study evaluating aluminum-based antiperspirant use in women receiving external beam RT for breast cancer found that antiperspirant use was not associated with any significant skin reaction compared to the control group [14]. Interestingly, in another study, aluminum-based antiperspirant use in breast cancer patients treated with pegylated liposomal doxorubicin was associated with a decreased incidence of grade 2 or 3 palmar-plantar erythrodysesthesia [15].
Skin care
Creams, ointments, lotions, and gels
Dermatitis, itchiness, xerosis, and erythema are common side effects of RT but can be ameliorated with the use of creams, ointments, lotions, and/or gels [1, 2]. Results from a large multi-institutional study found that thin or moderately applied topical agents have minimal effect on RT skin dose [56], negating suggestions that topicals should be avoided prior to RT. Application of topical vitamin E, RayGel, phytotherapic, urea, or antioxidant creams reduced onset and severity of RD [24,25,26, 57]. Urea-containing creams have shown benefit in preventing HFS during and following chemotherapy [25, 26]. Analgesic-containing gels, such as trolamine, may also play a role in alleviating acute RD by promoting wound healing. Trolamine use is associated with conflicting results, warranting additional studies of analgesic use for RD [23, 58].
Several published studies have found no benefits of certain emollients in acute skin reactions. Hydrosorb and Radiacare gel have been found to be ineffective in treating RD, while Biafine cream has demonstrated mixed results [19,20,21,22]. It is important for clinicians to counsel patients undergoing RT on the use of ineffective creams and gels, which may be found and ordered online.
Dressings
Dressings are often used to treat wounds from RT and since skin toxicity from RT can result in desquamation, many studies have evaluated the utility of dressings in the prevention and treatment of RD. Studies show that Mepitel film, silver nylon dressings, polymeric membrane dressings, 3 M Cavilon No-String Barrier film, Airwall film, Polyurethane hydrofilm, StrataXRT® silicone film, and Mepilex Lite dressing are effective at reducing the duration and frequency of RD [27,28,29,30,31,32,33,34,35]. One study found that a wet dressing, Hydrogel, resulted in a significant increase in healing time compared to a dry dressing, Tricotex [36].
Vitamin K
Epidermal growth factor receptor (EGFR) inhibitors such as cetuximab are associated with skin toxicity, namely, papulopustular (acneiform) eruptions following chemotherapy [59]. While there are currently no standard OTC available treatments in preventing EGFR inhibitor induced acneiform rash, vitamin K has been studied as a possible intervention [60].
Studies have found mixed results with the use of vitamin K cream, with a few studies reporting no reduction in the number of cetuximab induced papulopustular eruptions after use of vitamin K1 and vitamin K3 [44, 61]. However, there have been a few reports of lower proportions of grade 2 and grade 3 rash after use of vitamin K cream [43, 62]. Hofheinz et al. found that combination therapy did not decrease grade 2 + skin rash. There is currently no evidence-based recommendation to use vitamin K to prevent EGFR induced skin toxicity [60].
Natural products
Naturally-derived compounds are often incorporated into skincare regimens for their potential anti-inflammatory and antioxidant benefits for RD [39]. Three studies in this review evaluated the efficacy of aloe in reducing adverse skin reactions in patients undergoing RT. In two studies, aloe did not significantly reduce RD compared to either placebo or topical aqueous cream [37, 38]. In another study, adding aloe to a mild soap skin washing regimen demonstrated a protective effect as the cumulative radiation dose increased over time [63].
Other naturally derived compounds that have been investigated in trials include curcumin, Calendula, and Epigallocatechin-3-gallate (EGCG), a bioactive constituent of green tea, all of which demonstrate antioxidant properties [64,65,66]. Wolf et al. found that prophylactic treatment with topical curcumin was effective in minimizing skin reactions and pain for patients with high breast separation (i.e., larger breast size) at the end of RT [40]. In a RCT comparing topical Calendula cream versus standard of care (Sorbolene), no significant difference was observed for the prevention of RD [41]. Zhao et al. investigated whether EGCG can reduce the incidence of RD in patients after breast cancer surgery and found that EGCG prophylaxis significantly reduced both the incidence and severity of RD [42]. Cumulatively, these studies suggest that naturally-derived compounds with potential antioxidant properties may confer a protective effect against oxidative stress induced by free radicals during radiation treatment.
Antimicrobials and antiseptics
There are very few studies looking at non-prescription based antimicrobials or antiseptics, such as Gentian violet and chlorhexidine. Gentian violet has shown mixed results in treating RD [16, 17]. Chlorhexidine did not confer a protective benefit against infections in patients undergoing chemotherapy [18].
Hair care
Scalp cooling (SC) was the most commonly used hair care practice investigated in our review for chemotherapy-induced alopecia. In several trials, SC demonstrated efficacy in preventing chemotherapy-induced hair loss and may work better for patients receiving certain chemotherapies such as anthracyclines [48,49,50,51]. Additionally, prolonged post-infusion SC has been associated with better outcomes [52].
A few other studies investigated techniques such as the use of topicals and hair washing practices to prevent hair loss during chemotherapy. A study in patients with alopecia secondary to chemotherapy demonstrated that topical 2% minoxidil significantly reduced the period of baldness [47]. A novel topical containing a blend of four botanical ingredients (citrus, cocoa, guarana, and onion) was shown to increase hair density and thickness compared to baseline after 6 months of use [46]. Various hair washing techniques have not demonstrated any significant difference in hair loss compared to control [45]. Although avoidance of hair dye is a common recommendation, we did not find any articles that referenced hair dye or other chemicals.
Nail care
Nail toxicity has been a well-documented complication of chemotherapy, particularly that of taxane use, causing both functional impairment and psychological distress [67, 68]. Two studies investigated the use of cryotherapy to prevent docetaxel-induced hand and nail toxicity with conflicting results. While one trial with 41 patients found that onycholysis and skin toxicity were significantly reduced in the frozen glove protected hand [54], another trial with 21 patients found no significant difference between cutaneous hand toxicity in the gloved and non-gloved hands [53]. Further studies are needed to investigate whether cryotherapy can be used as an effective intervention to reduce taxane-induced nail and skin toxicity.
Morrison et al. investigated 2 interventions compared to standard of care for taxane-induced nail toxicity in women with early breast cancer [55]. Standard of care included lifestyle and hand hygiene practices aimed to prevent nail infection and damage including wearing household gloves when using chemicals, nail filing rather than cutting, and moisturizing hands around the fingernails. Two interventions included nail coverings (painting nails with dark nail varnish thought to prevent UV-induced damage) and Onicolife, a nail-specific medical advice consisting of anti-inflammatory and antiseptic compounds to protect tender and fragile nails). Compared to the use of dark nail varnish, standard care and the specialized Onicolife nail drops and nail oil were significantly associated with less nail toxicity [55].
Discussion
Patients undergoing cancer treatment often experience significant psychological distress and physical discomfort due to changes in their skin, hair, and nails. Unsubstantiated recommendations can add to this distress and hinder patients from resuming their normal lives during and post-cancer therapy, leading to unnecessary stress and anxiety. Clinicians play a pivotal role in assisting patients in maintaining their quality of life and sense of identity throughout the entire cancer treatment process. Therefore, it is imperative for clinicians to identify and counsel patients on evidence-based, effective, safe, and tolerable options for preventing and treating dermatologic disorders associated with cancer treatments. Additionally, it is important to individualize recommendations based on patient values and available evidence.
This systematic review underscores the efficacy of various OTC treatment modalities, with moisturizing creams and lotions having the highest quality of evidence and efficacy. Treatments with moderate quality of evidence and efficacy included antimicrobials and antiseptics, dressings, and natural products. It is important to acknowledge the varying quality of evidence for scalp cooling, a commonly used practice for chemotherapy-induced alopecia, necessitating larger clinical trials for a more comprehensive understanding of its efficacy and safety. Contradictory findings on the use of cryotherapy in taxane-induced nail toxicity as well as nail polishes and nail drops warrant further research in nail care practices. Importantly, recommendations on basic hygiene and routine care, such as avoiding certain practices, lacked support in the systematic review.
The results presented here must be interpreted with caution due to the variability in study sizes and the quality of study design. Certain treatment modalities were characterized by conflicting results and may not be generalizable to all patients. Different studies demonstrate short-term versus long-term benefits, emphasizing the importance of considering the duration of improvement for a given therapy. While the majority of studies in our review involved breast cancer patients, it is important to investigate evidence-based treatments in other cancer types owing to differences in the skin of various body regions. Compared to trials investigating evidence-based skin care regimens, there is a paucity of trials investigating those of hair and nail care.
In this systematic review, we summarized current evidence-based OTC recommendations in the literature as they pertain to skin, hair, and nail care management for adult patients before, during, and after cancer therapy, along with a quality of evidence assessment for each study. We hope that this review serves as a comprehensive guide for clinicians and patients to incorporate evidence-based recommendations and inspires further clinical trials to advance the field of supportive oncodermatology. This approach not only enhances quality of care but also fosters a sense of trust between healthcare providers and patients.
Data availability
All included studies and their respective data sources are referenced within the manuscript.
Abbreviations
- OTC :
-
Over the counter
- RD :
-
Radiation dermatitis
- RT :
-
Radiation therapy
- HFS :
-
Hand foot syndrome
- PRISMA :
-
Preferred reporting items for systematic reviews and meta-analyses
- LoE :
-
Level of evidence
- RCT :
-
Randomized controlled trialEGFR—epidermal growth factor receptor
- EGCG :
-
Epigallocatechin-3-gallate
- SC :
-
Scalp cooling
References
Alley E, Green R, Schuchter L (2002) Cutaneous toxicities of cancer therapy. Curr Opin Oncol 14(2):212–216. https://doi.org/10.1097/00001622-200203000-00012
Branzan AL, Landthaler M, Szeimies R-M (2005) [Skin changes with chemotherapy]. Hautarzt Z Dermatol Venerol Verwandte Geb 56(6):591–602. https://doi.org/10.1007/s00105-005-0971-0. (quiz 603)
Zarrabi K, Gemmill JAL, Safaee M, Baer L (2019) Managing dermatologic changes of targeted cancer therapy. J Fam Pract 68(6):334–340
Bouhanna P (2021) Alopecia after certain cancer treatments. Dermatologie Pratique. https://www.eyecare.fr/img/cms/outils/Hors%20se%CC%81rie-septembre%202021-version-anglaise-BD-2.pdf
Sherman DW, Walsh SM (2022) Promoting comfort: a clinician guide and evidence-based skin care plan in the prevention and management of radiation dermatitis for patients with breast cancer. Healthcare 10(8):1496. https://doi.org/10.3390/healthcare10081496
Bruner DW, Haas ML, Gosselin-Acomb TK (eds) (2004) Manual for Radiation Oncology Nursing Practice and Education, 3rd edn. Oncology Nursing Society, Pittsburgh, PA
Salvo N et al (2010) Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr Oncol Tor Ont 17(4):94–112. https://doi.org/10.3747/co.v17i4.493
Potthoff K et al (2011) Interdisciplinary management of EGFR-inhibitor-induced skin reactions: a German expert opinion. Ann Oncol 22(3):524–535. https://doi.org/10.1093/annonc/mdq387
Iacovelli NA et al (2020) Topical treatment of radiation-induced dermatitis current issues and potential solutions. Drugs Context 9:2020-4–7. https://doi.org/10.7573/dic.2020-4-7
Batchelor D (2001) Hair and cancer chemotherapy: consequences and nursing care–a literature study. Eur J Cancer Care (Engl) 10(3):147–163. https://doi.org/10.1046/j.1365-2354.2001.00272.x
Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg Lond Engl 8(5):336–341. https://doi.org/10.1016/j.ijsu.2010.02.007
Ball C, Sackett D, Phillips B, Haynes B, Straus S (2009) Levels of evidence and grades of recommendations Oxford Centre for Evidence-Based Medicine. [Online]. Available: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009. Accessed 15 Mar 2023
Roy I, Fortin A, Larochelle M (2001) The impact of skin washing with water and soap during breast irradiation: a randomized study. Radiother Oncol J Eur Soc Ther Radiol Oncol 58(3):333–339. https://doi.org/10.1016/s0167-8140(00)00322-4
Watson LC, Gies D, Thompson E, Thomas B (2012) Randomized control trial: evaluating aluminum-based antiperspirant use, axilla skin toxicity, and reported quality of life in women receiving external beam radiotherapy for treatment of Stage 0, I, and II breast cancer. Int J Radiat Oncol Biol Phys 83(1):e29-34. https://doi.org/10.1016/j.ijrobp.2011.12.006
Templeton AJ et al (2014) Prevention of palmar-plantar erythrodysesthesia with an antiperspirant in breast cancer patients treated with pegylated liposomal doxorubicin (SAKK 92/08). Breast Edinb Scotl 23(3):244–249. https://doi.org/10.1016/j.breast.2014.02.005
Mak SS et al (2005) A comparison of wound treatments in nasopharyngeal cancer patients receiving radiation therapy. Cancer Nurs 28(6):436–445. https://doi.org/10.1097/00002820-200511000-00005
Mak SS, Molassiotis A, Wan WM, Lee IY, Chan ES (2000) The effects of hydrocolloid dressing and gentian violet on radiation-induced moist desquamation wound healing. Cancer Nurs 23(3):220–229. https://doi.org/10.1097/00002820-200006000-00010
Yeomans A, Davitt M, Peters CA, Pastuszek C, Cobb S (1991) Efficacy of chlorhexidine gluconate use in the prevention of perirectal infections in patients with acute leukemia. Oncol Nurs Forum 18(7):1207–1213
Szumacher E et al (2001) Phase II study assessing the effectiveness of Biafine cream as a prophylactic agent for radiation-induced acute skin toxicity to the breast in women undergoing radiotherapy with concomitant CMF chemotherapy. Int J Radiat Oncol Biol Phys 51(1):81–86. https://doi.org/10.1016/s0360-3016(01)01576-0
Fisher J et al (2000) Randomized phase III study comparing Best Supportive Care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97–13. Int J Radiat Oncol Biol Phys 48(5):1307–1310. https://doi.org/10.1016/s0360-3016(00)00782-3
Bazire L et al (2015) Hydrosorb® versus control (water based spray) in the management of radio-induced skin toxicity: Results of multicentre controlled randomized trial. Radiother Oncol J Eur Soc Ther Radiol Oncol 117(2):229–233. https://doi.org/10.1016/j.radonc.2015.08.028
Gosselin TK, Schneider SM, Plambeck MA, Rowe K (2010) A prospective randomized, placebo-controlled skin care study in women diagnosed with breast cancer undergoing radiation therapy. Oncol Nurs Forum 37(5):619–626. https://doi.org/10.1188/10.ONF.619-626
Elliott EA et al (2006) Phase III Trial of an emulsion containing trolamine for the prevention of radiation dermatitis in patients with advanced squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Trial 99–13. J Clin Oncol Off J Am Soc Clin Oncol 24(13):2092–2097. https://doi.org/10.1200/JCO.2005.04.9148
Queiroz Schmidt FM, Serna González CV, Mattar RC, Lopes LB, Santos MF, de Gouveia Santos VLC (2022) Topical application of a cream containing nanoparticles with vitamin E for radiodermatitis prevention in women with breast cancer: A randomized, triple-blind, controlled pilot trial. Eur J Oncol Nurs Soc 61:102230. https://doi.org/10.1016/j.ejon.2022.102230
Hofheinz R-D et al (2015) Mapisal versus urea cream as prophylaxis for capecitabine-associated hand-foot syndrome: a randomized phase III trial of the AIO quality of life working group. J Clin Oncol Off J Am Soc Clin Oncol 33(22):2444–2449. https://doi.org/10.1200/JCO.2014.60.4587
Pardo Masferrer J et al (2010) Prophylaxis with a cream containing urea reduces the incidence and severity of radio-induced dermatitis. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex 12(1):43–48. https://doi.org/10.1007/s12094-010-0465-0
Yan J et al (2020) Mepitel Film is superior to Biafine cream in managing acute radiation-induced skin reactions in head and neck cancer patients: a randomised intra-patient controlled clinical trial. J Med Radiat Sci 67(3):208–216. https://doi.org/10.1002/jmrs.397
Vuong T et al (2004) Silver leaf nylon dressing to prevent radiation dermatitis in patients undergoing chemotherapy and external beam radiotherapy to the perineum. Int J Radiat Oncol Biol Phys 59(3):809–814. https://doi.org/10.1016/j.ijrobp.2003.11.031
Niazi TM et al (2012) Silver clear nylon dressing is effective in preventing radiation-induced dermatitis in patients with lower gastrointestinal cancer: results from a phase III study. Int J Radiat Oncol Biol Phys 84(3):e305-310. https://doi.org/10.1016/j.ijrobp.2012.03.062
Scott A (2014) Polymeric membrane dressings for radiotherapy-induced skin damage. Br J Nurs Mark 23(10):S24–S26-31. https://doi.org/10.12968/bjon.2014.23.Sup10.S24
Graham P et al (2004) Randomized, paired comparison of No-Sting Barrier Film versus sorbolene cream (10% glycerine) skin care during postmastectomy irradiation. Int J Radiat Oncol Biol Phys 58(1):241–246. https://doi.org/10.1016/s0360-3016(03)01431-7
Arimura T et al (2016) Effect of film dressing on acute radiation dermatitis secondary to proton beam therapy. Int J Radiat Oncol Biol Phys 95(1):472–476. https://doi.org/10.1016/j.ijrobp.2015.10.053
Schmeel LC et al (2018) Prophylactically applied Hydrofilm polyurethane film dressings reduce radiation dermatitis in adjuvant radiation therapy of breast cancer patients. Acta Oncol Stockh Swed 57(7):908–915. https://doi.org/10.1080/0284186X.2018.1441542
Chan RJ et al (2019) A single-blind, randomised controlled trial of StrataXRT® - A silicone-based film-forming gel dressing for prophylaxis and management of radiation dermatitis in patients with head and neck cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol 139:72–78. https://doi.org/10.1016/j.radonc.2019.07.014
Zhong W-H, Tang Q-F, Hu L-Y, Feng H-X (2013) Mepilex Lite dressings for managing acute radiation dermatitis in nasopharyngeal carcinoma patients: a systematic controlled clinical trial. Med Oncol Northwood Lond Engl 30(4):761. https://doi.org/10.1007/s12032-013-0761-y
Macmillan MS, Wells M, MacBride S, Raab GM, Munro A, MacDougall H (2007) Randomized comparison of dry dressings versus hydrogel in management of radiation-induced moist desquamation. Int J Radiat Oncol Biol Phys 68(3):864–872. https://doi.org/10.1016/j.ijrobp.2006.12.049
Hoopfer D et al (2015) Three-arm randomized phase III trial: quality aloe and placebo cream versus powder as skin treatment during breast cancer radiation therapy. Clin Breast Cancer 15(3):181-190.e1–4. https://doi.org/10.1016/j.clbc.2014.12.006
Heggie S et al (2002) A Phase III study on the efficacy of topical aloe vera gel on irradiated breast tissue. Cancer Nurs 25(6):442–451. https://doi.org/10.1097/00002820-200212000-00007
Siegel DM, Jakus J, Hooper D (2019) Topical natural products in managing dermatologic conditions: observations and recommendations. Cutis 103(4):233-236 E1;E2
Ryan Wolf J et al (2020) Utility of topical agents for radiation dermatitis and pain: a randomized clinical trial. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 28(7):3303–3311. https://doi.org/10.1007/s00520-019-05166-5
Siddiquee S et al (2021) Efficacy of topical Calendula officinalis on prevalence of radiation-induced dermatitis: A randomised controlled trial. Australas J Dermatol 62(1):e35–e40. https://doi.org/10.1111/ajd.13434
Zhao H et al (2022) Efficacy of epigallocatechin-3-gallate in preventing dermatitis in patients with breast cancer receiving postoperative radiotherapy: a double-blind, placebo-controlled, phase 2 randomized clinical trial. JAMA Dermatol 158(7):779–786. https://doi.org/10.1001/jamadermatol.2022.1736
Pinta F et al (2014) Pilot clinical trial on the efficacy of prophylactic use of vitamin K1-based cream (Vigorskin) to prevent cetuximab-induced skin rash in patients with metastatic colorectal cancer. Clin Colorectal Cancer 13(1):62–67. https://doi.org/10.1016/j.clcc.2013.10.001
Eriksen JG, Kaalund I, Clemmensen O, Overgaard J, Pfeiffer P (2017) Placebo-controlled phase II study of vitamin K3 cream for the treatment of cetuximab-induced rash. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 25(7):2179–2185. https://doi.org/10.1007/s00520-017-3623-x
Westbury C, Hines F, Hawkes E, Ashley S, Brada M (2000) Advice on hair and scalp care during cranial radiotherapy: a prospective randomized trial. Radiother Oncol J Eur Soc Ther Radiol Oncol 54(2):109–116. https://doi.org/10.1016/s0167-8140(99)00146-2
Kang D et al (2020) Impact of a topical lotion, CG428, on permanent chemotherapy-induced alopecia in breast cancer survivors: a pilot randomized double-blind controlled clinical trial (VOLUME RCT). Support Care Cancer Off J Multinatl Assoc Support Care Cancer 28(4):1829–1837. https://doi.org/10.1007/s00520-019-04982-z
Duvic M et al (1996) A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol 35(1):74–78. https://doi.org/10.1016/S0190-9622(96)90500-9
Mols F, van den Hurk CJ, Vingerhoets AJJM, Breed WPM (2009) Scalp cooling to prevent chemotherapy-induced hair loss: practical and clinical considerations. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 17(2):181–189. https://doi.org/10.1007/s00520-008-0475-4
Ridderheim M, Bjurberg M, Gustavsson A (2003) Scalp hypothermia to prevent chemotherapy-induced alopecia is effective and safe: a pilot study of a new digitized scalp-cooling system used in 74 patients. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 11(6):371–377. https://doi.org/10.1007/s00520-003-0451-y
Betticher DC et al (2013) Efficacy and tolerability of two scalp cooling systems for the prevention of alopecia associated with docetaxel treatment. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 21(9):2565–2573. https://doi.org/10.1007/s00520-013-1804-9
van den Hurk CJG, Breed WPM, Nortier JWR (2012) Short post-infusion scalp cooling time in the prevention of docetaxel-induced alopecia. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 20(12):3255–3260. https://doi.org/10.1007/s00520-012-1465-0
Komen MMC et al (2019) Prolonging the duration of post-infusion scalp cooling in the prevention of anthracycline-induced alopecia: a randomised trial in patients with breast cancer treated with adjuvant chemotherapy. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 27(5):1919–1925. https://doi.org/10.1007/s00520-018-4432-6
McCarthy AL, Shaban RZ, Gillespie K, Vick J (2014) Cryotherapy for docetaxel-induced hand and nail toxicity: randomised control trial. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 22(5):1375–1383. https://doi.org/10.1007/s00520-013-2095-x
Scotté F et al (2005) Multicenter study of a frozen glove to prevent docetaxel-induced onycholysis and cutaneous toxicity of the hand. J Clin Oncol Off J Am Soc Clin Oncol 23(19):4424–4429. https://doi.org/10.1200/JCO.2005.15.651
Morrison A et al (2022) A randomised controlled trial of interventions for taxane-induced nail toxicity in women with early breast cancer. Sci Rep 12(1):11575. https://doi.org/10.1038/s41598-022-13327-6
Baumann BC et al (2018) Assessing the validity of clinician advice that patients avoid use of topical agents before daily radiotherapy treatments. JAMA Oncol 4(12):1742–1748. https://doi.org/10.1001/jamaoncol.2018.4292
MikoEnomoto T, Johnson T, Peterson N, Homer L, Walts D, Johnson N (2005) Combination glutathione and anthocyanins as an alternative for skin care during external-beam radiation. Am J Surg 189(5):627–630. https://doi.org/10.1016/j.amjsurg.2005.02.001. (discussion 630–631)
Abbas H, Bensadoun R-J (2012) Trolamine emulsion for the prevention of radiation dermatitis in patients with squamous cell carcinoma of the head and neck. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 20(1):185–190. https://doi.org/10.1007/s00520-011-1110-3
Ayati A, Moghimi S, Salarinejad S, Safavi M, Pouramiri B, Foroumadi A (2020) A review on progression of epidermal growth factor receptor (EGFR) inhibitors as an efficient approach in cancer targeted therapy. Bioorganic Chem 99:103811. https://doi.org/10.1016/j.bioorg.2020.103811
Hofheinz R-D et al (2016) Recommendations for the prophylactic management of skin reactions induced by epidermal growth factor receptor inhibitors in patients with solid tumors. Oncologist 21(12):1483–1491. https://doi.org/10.1634/theoncologist.2016-0051
Jo J-C et al (2013) Topical vitamin K1 may not be effective in preventing acneiform rash during cetuximab treatment in patients with metastatic colorectal cancer. Eur J Dermatol EJD 23(1):77–82. https://doi.org/10.1684/ejd.2012.1899
Schimanski CC et al (2017) Dermatux: phase IV trial of Cetuximab plus FOLFIRI in first-line metastatic colorectal cancer receiving a pre-defined skin care. J Cancer Res Clin Oncol 143(6):1023–1034. https://doi.org/10.1007/s00432-017-2344-3
Olsen DL et al (2001) The effect of aloe vera gel/mild soap versus mild soap alone in preventing skin reactions in patients undergoing radiation therapy. Oncol Nurs Forum 28(3):543–547
Gupta SC, Patchva S, Aggarwal BB (2012) Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 15(1):195–218. https://doi.org/10.1208/s12248-012-9432-8
Korakhashvili A, Kacharava T, Kiknavelidze N (2007) Biochemical structure of Calendula officinalis. Georgian Med News 148–149:70–73
Nikoo M, Regenstein JM, Ahmadi Gavlighi H (2018) Antioxidant and Antimicrobial Activities of (-)-Epigallocatechin-3-gallate (EGCG) and its potential to preserve the quality and safety of foods. Compr Rev Food Sci Food Saf 17(3):732–753. https://doi.org/10.1111/1541-4337.12346
Miller KK, Gorcey L, McLellan BN (2014) Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol 71(4):787–794. https://doi.org/10.1016/j.jaad.2014.03.019
Childress J, Lokich J (2003) Cutaneous hand and foot toxicity associated with cancer chemotherapy. Am J Clin Oncol 26(5):435–436. https://doi.org/10.1097/01.coc.0000026486.56886.18
Funding
No external funding was received for this manuscript.
Author information
Authors and Affiliations
Contributions
Dr. McLellan had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Concept and design: BNM, BJ.
Acquisition, analysis, or interpretation data: BJ, LMP, SAR, ET.
Drafting of the manuscript: BJ, LMP, SAR.
Critical revision of the manuscript for important intellectual content: BJ, LMP, SAR, BNM.
Statistical analysis: Not applicable.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Disclosure
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Javdan, B., Pattison, L.M., Rangu, S.A. et al. The validity of over-the-counter skin, hair, and nail recommendations for adult patients with cancer: A systematic review. Support Care Cancer 32, 577 (2024). https://doi.org/10.1007/s00520-024-08735-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08735-5