Abstract
Purpose
A MASCC/ISOO Clinical Practice Statement (CPS) is aimed at generating a concise tool for clinicians that concentrates practical information needed for the management of oral complications of cancer patients. This CPS raises awareness to the prevention of medication-related osteonecrosis of the jaw (MRONJ) in patients with breast cancer treated with adjuvant bone-modifying agents (BMA).
Methods
This CPS was developed based on a critical evaluation of the literature followed by a structured discussion of a group of leading experts, members of the Oral Care Study Group of MASCC/ISOO. The information is presented in the form of succinct bullets and tables to generate a short manual about the best standard of care.
Results
In patients treated with adjuvant BMA, dento-alveolar surgery poses a moderate risk for MRONJ that ranges between the high risk for MRONJ in patients with metastatic breast cancer and the low risk for MRONJ in patients with osteoporosis. Existing MRONJ guidelines serve as a starting point for adjuvant BMA use. Urgent procedures should be delivered without delay using the accepted precautions to prevent MRONJ. If elective surgery is considered, the individual risk for MRONJ following surgery should be assessed according to common risk factors.
Conclusion
Prevention of MRONJ in primary breast cancer patients treated with adjuvant BMA requires risk–benefit assessment; collaboration between the medical team, dental professional, and patient; and patient-specific tailored dental treatment planning. The patient should be informed about this risk. Additional research is needed to define optimal MRONJ care for this population.
Avoid common mistakes on your manuscript.
Introduction
Treatment with bone-modifying agents (BMA) such as bisphosphonates (BP) and denosumab is associated with a risk of medication-related osteonecrosis of the jaw (MRONJ) [1]. Even with proper management, MRONJ may persist and affect quality of life and oral function [2]. Different patient populations may have a variable risk for MRONJ [2].
Adjuvant BP therapy is used in patients with non-metastatic breast cancer and correlates with a modest improvement in overall survival [3]. A recent joint guidelines paper from the American Society of Clinical Oncology (ASCO) and Ontario Health (OH) discusses the use of adjuvant BP for all primary breast cancer patients who are postmenopausal (natural or therapy-induced) [4]. Breast cancer affects approximately 300,000 new patients per year in the USA [5] and about 2,000,000 globally [6], and the majority are postmenopausal; therefore, the use of adjuvant BP may increase the incidence of MRONJ.
According to the ASCO/OH guidelines, the most robust evidence supports the following therapeutic options (listed alphabetically):
-
Clodronate—1600 mg per os daily for 2–3 years
-
Ibandronate—50 mg per os daily for 3 years
-
Zoledronic acid (zoledronate, ZA)—4 mg intravenous once every 6 months for 3 years
-
ZA—4 mg intravenous once every 3 months for 2 years
To date, there is no formal society recommendation supporting the use of denosumab for this indication. Furthermore, in current studies of denosumab for adjuvant therapy in primary breast cancer, the dose used was either equivalent to osteoporosis dose or to bone metastasis dose [7]. Both doses are addressed in existing guidelines [1, 2]. Therefore, this paper will only refer to BP.
The target population of adjuvant BP is being treated for cure, and these patients are expected to have a long life expectancy compared to metastatic breast cancer. Thus, dentists increasingly will be called upon to treat these patients and the medical team needs to be aware of possible implications on the patient’s future dental care.
In two out of the four ASCO/OH BP regimens, the recommended dose for adjuvant BP in patients with primary breast cancer is similar to the dose recommended for metastatic breast cancer (clodronate and ibandronate regimens; see Table 1). Therefore, these patients treated with the ibandronate regimen should be addressed according to the existing guidelines for dental management of metastatic breast cancer patients [1, 2]. Clodronate is not in common use nowadays or not approved for use in many countries, including in the USA. Accordingly, ibandronate and clodronate are excluded from the discussion in this publication. Notwithstanding, the exclusion of ibandronate and clodronate does not suggest that they are not associated with increased risk for MRONJ.
In the remaining two ASCO/OH BP regimens (ZA regimens; see Table 1), the annual dose of this BP (8 mg/year or 16 mg/year) is lower than the annual dose of this BP in metastatic disease (16–48 mg/year). Furthermore, these regimens for the adjuvant ZA in primary breast cancer are administered for a limited duration (2–3 years) relative to the BP regimens in metastatic cancer which are often longer (typically indefinite) [8]. Therefore, the cumulative dose is expected to be lower in primary breast cancer administered adjuvant ZA. Accordingly, this CPS will focus on the adjuvant ZA regimens for primary breast cancer, which may have new implications related to MRONJ.
In 2019, the Multinational Association of Supportive Care in Cancer (MASCC), the International Society of Oral Oncology (ISOO), and the American Society of Clinical Oncology (ASCO) published clinical practice guidelines for the prevention and management of MRONJ in cancer patients [1]. This comprehensive publication focused on patients with metastatic cancer or multiple myeloma, and did not refer to patients treated with adjuvant BP. Evidence regarding MRONJ in patients with primary breast cancer is scarce resulting in a clinical gap regarding the prevention of MRONJ in patients treated with adjuvant BP. There are two unique aspects related to this patient population—dental care before and during adjuvant BP and long-term dental care. As patients age, the need for complex dental procedure may increase, and the risk for MRONJ is higher with invasive interventions. Conversely, the risk for MRONJ may decrease after the cessation of the adjuvant BP. Therefore, a working group of the Oral Care Study Group (OCSG) of MASCC/ISOO developed this clinical practice statement (CPS) to suggest an approach to patients with primary breast cancer treated with BP in the adjuvant setting.
Objectives
This study aims to raise awareness to the risk for MRONJ in patients with breast cancer treated with adjuvant ZA, to provide risk-mitigating strategies, and, additionally, to outline the main considerations in the dental management of these patients in order to prevent MRONJ.
Methods
This CPS is based on a compilation of expert opinions with a high-quality review of the literature. The literature search was conducted on PubMed on data pertinent to MRONJ and adjuvant BP in the timeframe up to January 1, 2023. During the development of the manuscript, point questions that deemed a closer look were generated, and a literature search was done to ensure the accuracy of the information. The CPS was discussed internally by a working group of OCSG members who are experts on the topic of MRONJ, and then reviewed by two independent boards: the ISOO Advisory Board and the MASCC Guidelines Committee. The Statement follows the MASCC/ISOO Guidelines Policy.
Clinical relevance and practical considerations
-
Multi-disciplinary coordination and patient education
-
Dental care should be coordinated between the dentist and the oncology team to ensure that necessary procedures are undertaken prior to the initiation of adjuvant BP. An open discussion between the patient, the oncologist, and the dentist regarding the risks, benefits, and extent of the prophylactic dental treatment is advised in order to achieve optimal care and a better quality of life. As there is no information if delay in starting adjuvant BP decreases its efficacy in the prevention of metastases, delay of dental care should be minimal. ASCO/OH suggests initiating BP within 3 months of definitive surgery or within 2 months of completion of adjuvant chemotherapy [4].
-
Given that the benefit of adjuvant BP treatment to the patient’s overall survival is modest [4], the value of prescribing the adjuvant BP should be weighed relative to the likelihood for future invasive dental procedures within the immediate and long-term time period.
-
Patients should be educated regarding the risk for MRONJ (Table 2) and the importance of dental evaluation prior to adjuvant BP treatment and thereafter.
Table 2 Rate of MRONJ in adjuvant bisphosphonate protocols for primary breast cancer -
The oncology care team should advise the patients to inform the dentist about being treated with BP, either planned, current, or prior doses, regardless of the type of dental procedure. This is of utmost importance, since patients may omit reporting treatments given intravenously in an ambulatory care setting biannually. This communication augments the information flow about all other comorbidities and medication lists. The dental care team should be aware that patients with non-metastatic breast cancer may be treated with BP.
-
The dental care team should advise the patients to have routine dental check-ups and periodontal maintenance throughout the adjuvant BP treatment and following its completion. Patients should be encouraged to practice meticulous daily oral hygiene as a preventive measure. For more details on the information that should be delivered to patients prior to BP therapy, please see Table 4 in the MASCC/ISOO/ASCO guidelines paper [1].
-
Patients are advised to consult with an experienced dental specialist prior to high-risk dento-alveolar procedures or when in doubt about the dental treatment planning.
-
-
Treatment plan considerations
-
Dental treatment prior to the initiation of adjuvant BP should be individualized according to the patient’s risk factors. The presence of a likely risk factor for MRONJ (such as steroid use) may drive a more deterministic dento-alveolar surgical approach prior to the initiation of the adjuvant BP [2]. Other possible risk factors for MRONJ that have inconsistent evidence include diabetes, age, smoking, anemia, and certain concurrent medications, such as angiogenesis inhibitors, periodontal disease, and the presence of dentures [1, 2, 14, 15]. Accumulating dose of BP should also play a role in the risk assessment [2]. Therefore, extension of the adjuvant BP beyond the ASCO recommendation or BMA treatment prior to the adjuvant BP for osteoporosis poses an additional risk.
-
Given the lower annual cumulative dose of adjuvant ZA in primary breast cancer patients (8 or 16 mg/year) relative to ZA dose in metastatic breast cancer (16–48 mg/year), the risk for MRONJ is likely lower in the former group. On the other hand, the risk for MRONJ with the adjuvant BP regimen is probably higher than in osteoporosis patients (5 mg/year) [16]. Therefore, the clinical approach for dento-alveolar surgery should be at a mid-level strategy, and consider the extent of total exposure of ZA.
-
This patient population may be treated with additional pharmacological agents. As new therapies are introduced in the market and the profile of their adverse effects is being revealed, modifications to the dental treatment plan may be needed. In particular, the clinicians should be aware of the patient’s immune status and possible risk for oral infections.
-
For more details on the dental preparations of patients prior to BP therapy, please see Table 3 in the MASCC/ISOO/ASCO guidelines paper [1].
-
Geographic variation in health and or dental insurance may impact the implementation of this CPS. Attempts should be made to address dental needs within the economic challenges.
-
-
Dento-alveolar surgery for patients on adjuvant BP
-
Urgent procedures should be delivered without delay. Antibiotics should be considered if signs of infection/inflammation are observed. Standard postoperative practices to prevent secondary infections should be employed.
-
The evidence of the risk for MRNOJ following a dento-alveolar surgery in adjuvant BP regimens is scarce. It is unknown whether elective procedures should be delivered during the 2–3 years of the adjuvant BP regimens. Therefore, the clinician should consider the necessity of dento-alveolar procedures carefully.
-
Elective dento-alveolar surgery in patients taking adjuvant BP is estimated to pose a moderate risk for MRONJ that ranges between the high risk for MRONJ in patients with metastatic breast cancer and the low risk for MRONJ in patients with osteoporosis.
-
In elective dento-alveolar surgery, the risk should be stratified individually according to common risk factors (see Box).
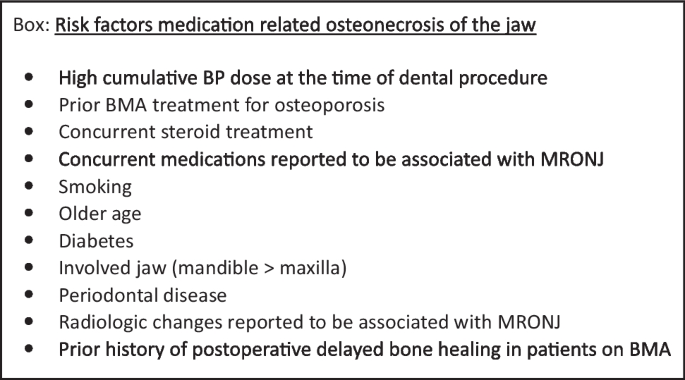
-
In patients with a higher cumulative dose of BP or multiple risk factors for MRONJ, a more conservative dento-alveolar surgical approach is advised, as in patient taking the ZA dose for metastatic cancer [2].
-
The optimal timing of an elective bone invasive procedure in relationship to BP dosing is unknown. If a dento-alveolar surgery is deemed necessary, and in order to improve wound healing and decrease the risk for MRONJ, it is speculated that it is preferable to:
-
Perform the procedure as far as possible from the last BP dose.
-
After the dento-alveolar procedure, hold the next BP dose until adequate osseous healing is observed.
-
-
For more details on the considerations related to surgical site manipulation, please see the AAOMS position paper [2].
-
-
Dento-alveolar surgery for patients who completed adjuvant BP
-
There are insufficient data at this time to allow a recommendation. The risk for MRONJ may decrease after the completion of adjuvant BP; however, as the clinical experience and literature increase, the clinical approach will get clear.
-
-
Management
Data availability
No datasets were generated or analyzed during the current study.
References
Yarom N, Shapiro CL, Peterson DE, Van Poznak CH, Bohlke K, Ruggiero SL, Migliorati CA, Khan A, Morrison A, Anderson H, Murphy BA, Alston-Johnson D, Mendes RA, Beadle BM, Jensen SB, Saunders DP (2019) Medication-related osteonecrosis of the jaw: MASCC/ISOO/ASCO Clinical Practice Guideline. J Clin Oncol 37(25):2270–2290
Ruggiero SL, Dodson TB, Aghaloo T, Carlson ER, Ward BB, Kademani D (2022) American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 update. J Oral Maxillofac Surg 80(5):920–943
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2015) Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 386(10001):1353–1361
Eisen A, Somerfield MR, Accordino MK, Blanchette PS, Clemons MJ, Dhesy-Thind S, Dillmon MS, D’Oronzo S, Fletcher GG, Frank ES, Hallmeyer S, Makhoul I, Moy B, Thawer A, Wu JY, Van Poznak CH (2022) Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: ASCO-OH (CCO) Guideline update. J Clin Oncol 40(7):787–800. https://doi.org/10.1200/JCO.21.02647
Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA Cancer J Clin 73(1):17–48
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Gnant M, Frantal S, Pfeiler G et al (2022) Long-term outcomes of adjuvant denosumab in breast cancer. NEJM Evid 1(12):VIDoa2200162. https://doi.org/10.1056/EVIDoa2200162
Van Poznak C, Somerfield MR, Barlow WE, Biermann JS, Bosserman LD, Clemons MJ, Dhesy-Thind SK, Dillmon MS, Eisen A, Frank ES, Jagsi R, Jimenez R, Theriault RL, Vandenberg TA, Yee GC, Moy B (2017) Role of bone-modifying agents in metastatic breast cancer: an American Society of Clinical Oncology-Cancer Care Ontario Focused Guideline Update. J Clin Oncol 35(35):3978–3986
Gralow JR, Barlow WE, Paterson AHG, M’iao JL, Lew DL, Stopeck AT, Hayes DF, Hershman DL, Schubert MM, Clemons M, Van Poznak CH, Dees EC, Ingle JN, Falkson CI, Elias AD, Messino MJ, Margolis JH, Dakhil SR, Chew HK, Dammann KZ, Abrams JS, Livingston RB, Hortobagyi GN (2020) Phase III randomized trial of bisphosphonates as adjuvant therapy in breast cancer: S0307. J Natl Cancer Inst 112(7):698–707. https://doi.org/10.1093/jnci/djz215
Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Heck D, Menzel C, Jakesz R, Seifert M, Hubalek M, Pristauz G, Bauernhofer T, Eidtmann H, Eiermann W, Steger G, Kwasny W, Dubsky P, Hochreiner G, Forsthuber EP, Fesl C, Greil R, Austrian Breast and Colorectal Cancer Study Group, Vienna, Austria (2011) Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol 12(7):631–41
Perrone F, De Laurentiis M, De Placido S, Orditura M, Cinieri S, Riccardi F, Ribecco AS, Putzu C, Del Mastro L, Rossi E, Tinessa V, Mosconi AM, Nuzzo F, Di Rella F, Gravina A, Iodice G, Landi G, Pacilio C, Forestieri V, Lauria R, Fabbri A, Ibrahim T, De Maio E, Barni S, Gori S, Simeon V, Arenare L, Daniele G, Piccirillo MC, Normanno N, de Matteis A, Gallo C (2019) Adjuvant zoledronic acid and letrozole plus ovarian function suppression in premenopausal breast cancer: HOBOE phase 3 randomised trial. Eur J Cancer 118:178–186
Friedl TWP, Fehm T, Müller V, Lichtenegger W, Blohmer J, Lorenz R, Forstbauer H, Fink V, Bekes I, Huober J, Jückstock J, Schneeweiss A, Tesch H, Mahner S, Brucker SY, Heinrich G, Häberle L, Fasching PA, Beckmann MW, Coleman RE, Janni W, Rack B (2021) Prognosis of patients with early breast cancer receiving 5 years vs 2 years of adjuvant bisphosphonate treatment: a phase 3 randomized clinical trial. JAMA Oncol 7(8):1149–1157
Coleman RE, Collinson M, Gregory W, Marshall H, Bell R, Dodwell D, Keane M, Gil M, Barrett-Lee P, Ritchie D, Bowman A, Liversedge V, De Boer RH, Passos-Coelho JL, O’Reilly S, Bertelli G, Joffe J, Brown JE, Wilson C, Tercero JC, Jean-Mairet J, Gomis R, Cameron D (2018) Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04). J Bone Oncol 13:123–135
Kizub DA, Miao J, Schubert MM, Paterson AHG, Clemons M, Dees EC, Ingle JN, Falkson CI, Barlow WE, Hortobagyi GN, Gralow JR (2021) Risk factors for bisphosphonate-associated osteonecrosis of the jaw in the prospective randomized trial of adjuvant bisphosphonates for early-stage breast cancer (SWOG 0307). Support Care Cancer 29(5):2509–2517. https://doi.org/10.1007/s00520-020-05748-8
Van Poznak CH, Unger JM, Darke AK, Moinpour C, Bagramian RA, Schubert MM, Hansen LK, Floyd JD, Dakhil SR, Lew DL, Wade JL 3rd, Fisch MJ, Henry NL, Hershman DL, Gralow J (2021) Association of osteonecrosis of the jaw with zoledronic acid treatment for bone metastases in patients with cancer. JAMA Oncol 7(2):246–254. https://doi.org/10.1001/jamaoncol.2020.6353
Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D (2019) Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* Clinical Practice Guideline. J Clin Endocrinol Metab 104(5):1595–1622
Acknowledgements
The OCSG of MASCC/ISOO is grateful to the ISOO Advisory Board and MASCC Guidelines Committee, which reviewed this statement and provided valuable feedback.
Funding
Open access funding provided by Tel Aviv University.
Author information
Authors and Affiliations
Contributions
N. Yarom and S. Elad contributed to the study conception and design. The first draft of the manuscript was written by N. Yarom and S. Elad. Specific material preparations were performed by G. Ottaviani, Y. Matsuda, N. Yarom, and S. Elad. C. H. Van Poznak had a special contribution to the manuscript writing. J. B. Epstein and C. Migliorati critically reviewed and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
N. Yarom, G. Ottaviani, Y. Matsuda, and S. Elad reported no relevant financial or non-financial interests to disclose. C. H. Van Poznak received funding from Bayer for a clinical trial unrelated to the subject matter. J. B. Epstein is a consultant for Rakuten, Sanotize Inc., Janssen, and Nielsen Inc. C. Migliorati is an adjudicator for Amgen Inc. J. B. Epstein is also the Associate Editor-in-Chief for Supportive Care in Cancer.
Disclaimer
The MASCC/ISOO OCSG Statements have been developed to facilitate expert opinion-based management of oral complications of cancer and cancer therapy, where high-quality evidence is lacking. Clinicians should use their judgment when making treatment decisions for individual patients. Statement authors and the MASCC/ISOO do not guarantee or take responsibility for the clinical outcomes in individual patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yarom, N., Van Poznak, C.H., Epstein, J.B. et al. MASCC/ISOO Clinical Practice Statement: Adjuvant bone-modifying agents in primary breast cancer patients - prevention of medication-related osteonecrosis of the jaw. Support Care Cancer 32, 547 (2024). https://doi.org/10.1007/s00520-024-08687-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08687-w


