Abstract
Purpose
Patients with advanced pancreatic and biliary tract cancer (aPBC) frequently suffer from high symptom burden. Exercise can reduce treatment side effects and improve patient-related outcomes (PROMs). However, evidence from prospective studies regarding feasibility and efficacy in advanced settings are sparse. The primary aim of this prospective, randomized-controlled study was to evaluate the feasibility and effects of exercise (ET) in patients with aPBC.
Methods
Patients with aPBC beyond first-line therapy were randomized according to the minimization procedure with stratification by gender, age, and loss of body weight in the past six months. The intervention group (IG) completed 3 training units/week for 8 weeks (1x supervised strength sessions, 2x individualized home-based sessions). Control group (CG) received recommendations on physical activity during cancer.
Results
41 patients (stage IV pancreatic or biliary tract cancer) were included no adverse events related to exercise occurred during the trial. Physical function increased significantly in IG in 5 out of 7 physical domains. Comparison of IG and CG at 8 weeks (t2) showed significant differences in favour of IG in leg press (p=0.001), bench press (p=0.011), sit-to-stand (p=0.001) and crunch (0.006). Constipation revealed a significant difference in favour of IG at t2 (p=0.033). Quality of life stabilized/increased in IG during the study period compared to a decrease in CG. Throughout/Over the 8 weeks, fatigue notably reduced in the IG (p=0.028).
Conclusion
Exercise is safe and feasible in patients with aPBC undergoing further line therapy. Significant improvements in physical functioning and increased quality of life were achieved.
German Clinical Trials Register ID: DRKS00021179; Registration date 15.05.2020
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advanced pancreatic ductal adenocarcinoma (PDAC) and biliary tract cancer (BTC) exhibit poor overall survival and low 5-year survival rates [1, 2]. Collectively referred to as advanced pancreatobiliary cancers (aPBC), both entities are typically diagnosed at advanced stages with limited surgical options. Despite growing molecular insights and systemic therapies, (multi-)chemotherapy remains the primary approach [3,4,5,6]. These patients often bear a substantial symptom burden, including fatigue [7, 8], muscle weakness [9], nausea, anxiety [10], depression, and tumor-associated cachexia [11], affecting nearly 80% of cases. Cancer cachexia, a metabolic syndrome common in aPBC patients, is associated with muscle wasting, fatigue, and non-reversible weight loss despite conventional treatments [12]. Its mechanisms involve altered cytokine levels, inflammation, oxidative stress, and metabolism changes [13], contributing to diminished quality of life and survival [14,15,16]. In cancer care, exercise has shown promise, reducing fatigue [17], mitigating depression, anxiety, and sleep issues [18], and potentially enhancing quality of life [19]. Beneficial effects on body composition are seen, as well as potential anti-inflammatory effects against cancer cachexia [20]. However, exercise's feasibility and efficacy beyond first-line treatment in aPBC is underexplored, with limited prospective evidence [21,22,23], lacking clarity on optimal types, doses, and timing, considering the heterogeneity of this population. The P-move study aimed to assess feasibility of exercise beyond first-line chemotherapy in patients with aPBC, evaluating effects on physical function and patient-reported outcomes.
Materials and methods
Trial design
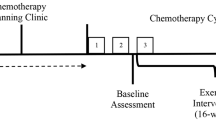
P-move was a single-center randomized trial. Patients were randomly assigned to the intervention group (IG), receiving supervised and unsupervised strength training for 8 weeks, or the control group (CG), receiving standard care.
Recruitment and assignment
Forty-one patients receiving palliative oncological treatment were enrolled from July 2020 to January 2023. Inclusion criteria were age ≥ 18 years, diagnose of advanced pancreatobiliary carcinomas (aPBC) (Stage III-IV), switching beyond first-line chemotherapy after progression of disease, life expectancy of at least 3 months, Eastern Cooperative Oncology Group (ECOG) status ≤ 2 and absence of serious comorbidities contraindicating exercise, such as severe cardiopulmonary disease, (EF<45%, heart failure NYHA III-IV, severe respiratory partial or global failure, uncontrolled hypertension), uncontrolled central nervous system metastases or bone metastases with risk of pathologic fracture (trial protocol DRKS00021179). After giving informed consent, each patient underwent baseline assessments prior to randomization using the principle of minimization, considering age, sex, and recent weight loss. Personal and medical data were pseudonymized with identification codes. Blinding of participants and scientist wasn't feasible due to the intervention's nature.
Equity, diversity, and inclusion statement
The author group consists of male and female investigators from different disciplines. Our study population included both male and female subjects with aPDAC or aBTC.
Intervention
The intervention consisted of 3 exercise sessions per week for a duration of 8 weeks. One training session per week was delivered supervised by a qualified exercise physiologist and 2 training sessions were unsupervised at home. The focus of the intervention was resistance exercise targeting the main muscle groups. Each supervised exercise session consisted of a 5-minute warm-up on a bicycle ergometer and 40 minutes of resistance exercise focused on hypertrophy. Supervised resistance training consisted of the following exercises: leg press, bench press, latissimus pulldown, crunch and back extension. Each exercise contained 3 sets of twelve repetitions with two-minute breaks between sets. The initial load of resistance was determined using the hypothetical one-repetition maximum (h-1RM) regarding the Brzycki formula [24]. All participants started with 50% of the h-1RM to ensure safety. Progressions during the 8-week period were applied depending on the participants’ feedback and performance the week before. Since our patient group was fragile due to the tumor burden, a reduction in exercise load was necessary if patients reported fatigue or muscle soreness.
Home-based exercise consisted of bodyweight and resistance band exercises tailored to individual needs, with an included resistance band (Supplementary file 1).
Usual care/control group
The CG attended a single exercise counselling session, offering general guidance on physical activity during cancer. Recommendations aligned with current scientific insights [25, 26] (Supplementary file 2).
Primary outcomes
P-move’s primary aim was to evaluate the feasibility of tailored strength exercises beyond first-line treatment for aPBC patients. This encompassed recruitment and dropout rates, adherence to both supervised and home-based sessions, and exercise safety. All withdrawals were considered as drop-outs, with reasons noted. The exercise physiologist monitored adherence to the supervised sessions. For home-based exercise, patients reported completion frequency weekly during supervised sessions. Adverse events (related to therapy or exercise) were evaluated using Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.
Secondary outcomes
Secondary outcomes were differences in physical function (h-1RM of leg press, bench press, crunch, back extension, latissimus pulldown, handgrip strength and 1-minute-Sit-to-Stand (1-m-STST) within groups and between groups measured at baseline (t0), after 4 weeks (t1) and after 8 weeks (t2). Furthermore, we evaluated the differences within and between groups in quality of life with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ-C30) and Physical Activity, Exercise, and Sport Questionnaire (BSA) [27, 28].
Statistical analysis
The trial’s pilot nature prompted us to estimate sample size following Kieser and Wassmer’s rules of thumb [29] for two-armed pilot trials. The primary endpoint assessed exercise feasibility beyond first-line treatment, encompassing recruitment, adherence, drop-outs, and safety. Descriptive statistics included all participants. Baseline characteristics were presented means, medians, ranges (quantitative variables), and numbers/percentages (categorical variables). Non-parametric tests (Mann-Whitney U Test and Wilcoxon rank-sum test) utilized for intergroup and intragroup outcomes. Statistical analysis was performed using SPSS version 29 (IBM, USA) with an intention-to-treat approach. Significance was set at p < 0.05 (2-tailed) across all tests.
Registrations and approvals
This study was performed in line with the principles of the Declaration of Helsinki. The Ethics Committee of the Medical Faculty University of Duisburg-Essen approved this study prior to participant inclusion (19-8843-BO).
Results
Recruitment
From July 2020 to January 2023, 84 patients from the West German Cancer Center were screened whereof 52 (61%) fulfilled inclusion criteria. In total, 41 patients (78%) participated as described in Fig. 1.
P-move participants exclusively had stage IV cancer (100%). They averaged 58.7 years (SD 12.08) with a normal BMI of 23.7 (SD 4.7). Patients had received an average of 14.58 chemotherapy cycles (SD 11.08) over 2.5 treatment lines (SD 1.0) with enrolment. We enrolled 16 patients (39%) with over 5% weight loss in the last 6 months, constituting the cachexia group. Table 1 outlines the baseline characteristics by group.
Feasibility
Recruitment rate
Of the 84 aPBC patients screened (Fig. 1: CONSORT Flow Diagram), 32 were ineligible, mostly due to ECOG score ≥ 2 (n=23) or death between screening and inclusion (n=7). Among the 52 eligible patients, not participating was mainly due to distance from the study center (n=6), unexplained refusal (n=2), lack of exercise interest (n=1), existing physical activity (n=1), or excessive symptom burden from antitumor therapy (n=1). Overall recruitment reached 78%.
Drop-out rate
At the final assessment, 31 out of 41 patients provided complete data, resulting in a dropout rate of 24% (n=10). Reasons included disease progression/decline in health (n=6), cancer-related death (n=3), and a change in treatment hospital (n=1). Dropouts were evenly distributed between IG and CG, each with 5 patients, all of whom had PDAC. In comparison between completers and drop-outs, blood sampling at inclusion revealed significantly higher inflammatory parameters (interleukin 6 U= 61.50, Z=-2.537, p=0.011; neutrophil/lymphocyte ratio (NLR) U=42.00, Z=-3.158, p<0.00; C-reactive protein U=46.00, Z=-3.082, p=0.002) in dropouts. Lower metabolic parameters (calcium U=77.50, Z=-2.01, p=0.044), organ function (e.g., liver and thyroid) (alkaline phosphatase U=52.50, Z=-2.18, p=0.005, gamma-glutamyltransferase U=47.00, Z=-2.996, p=0.003, L-lactate dehydrogenase U=66.50, Z=-2.37, p=0.018, albumin U=28.00, Z=-3.623, p<0.001). There were lowered triiodothyronine values (U=33.50, Z=-0.421, p<0.001) and higher thyroxine values (U=65.00, Z=-2.414, p=0.016) at baseline measurement.
Adherence to exercise
IG participants completed 75% of scheduled exercise sessions (273/360) across 8 weeks, ranging from eight to twenty-four on an individual basis. Adherence stood at 83% (80/90) for supervised sessions and 71% (172/240) for home-based sessions. Four patients (2 PDAC, 2 BTC) reported <75% training perception, while 11 achieved >75% adherence. Predominantly, negative tumor therapy effects were reasons for non-participation.
Adverse events
Table 2 shows the overall adverse events during the trial. The most common adverse events were related to the cytotoxic side effects of antitumor therapy, followed by tumor-related adverse events and infectious disease.
Across the IG, 39 adverse events were observed during the trial, compared to 54 in the CG. Both groups reported 6 adverse events rated as >3 in severity. Three patients entered the study with pre-existing shoulder issues: two with shoulder joint osteoarthritis and 1 with inflammation, resulting in personalized exercise adjustments. The interdisciplinary team deemed the likelihood of adverse events being exercise-related as low.
Secondary outcomes
Physical function
All physical domains are presented in Fig. 2, and significant differences within and between groups are shown at all measurement points.
Handgrip strength
For left handgrip strength, there were no significant intergroup differences at t0 (U=113.00, Z=-0.277, p=0.782), t1 (U=103.00, Z=-0.087, p=0.30), and t2 (U=103.00, Z=-0.672, p=0.502). Likewise, right handgrip strength showed no significant differences: t0 (U=113.00, Z=-0.277, p=0.782), t1 (U=97.00, Z=-0.349, p=0.727), and t2 (U=100.00, Z=-0.791, p=0.429). Delta changes for left handgrip: CG mean -1.00 kg. (CI -3.43 – 1.42), IG mean change 1.31 kg. (CI -1.69 – 4.32). Delta changes for right handgrip: CG mean -0.71 kg. (CI -2.90 – 1.48), IG mean change 1.96 kg. (CI -1.21 – 5.15).
One-minute Sit-to-Stand-Test
The functional 1-m-STST showed a significant difference in favour of the IG group at baseline t0 (U=58.50, Z=-2.437, p=0.015), t1 (U=35.00, Z=-3.062 p=0.002) and t2 (U=32.50, Z= -3,464, p < 0.001). Within-group comparisons showed a significant increase in IG (t0 median 24, t2 median 25 z= -3.294 p=0.001). Delta change of CG mean 0 repetitions confidence interval (CI) -2.54 – 2.54 compared to IG mean change 4.33 repetitions (CI 2.35 – 6.31).
Leg press
Baseline measurements showed no significant differences. However, after 4 weeks of exercise, leg press strength significantly favored the intervention group (IG) (U=54.00, Z=-2.228, p=0.026). At the final measurement (t2), IG demonstrated a substantial advantage over the CG (U=32.00, Z=-3.481, p<0.001). These trends were consistent in within-group comparisons, where IG exhibited a significant increase over the 8-week training (t0 median 74.56, t2 median 97.51, Z=-3.160, p=0.002). Delta changes in leg press CG mean -6.86 kg. (CI -15.92 – 2.20), IG mean change 22.27 kg. (CI 14.37 – 30.17).
Bench press
No intergroup difference was noted in baseline measurements (U=32.00, Z=-0.686, p=0.498). However, after 4 weeks of exercise, a significant advantage favored the IG over the CG (U=48.00, Z=-2.281, p=0.023). This pattern persisted at t2, favoring the IG (U=50.50, Z=-2.558, p=0.011). Within the exercise group, a noteworthy increase over time was seen (t0 median 10.63, t2 median 16.31, Z=-3.107, p=0.002). Delta changes in chest press strength CG mean 0.34 kg. (CI -1.82 – 2.50), IG mean change 5.20 kg. (CI 2.69 – 7.70).
Latissimus-Pulldown
There was no significant difference between groups at t0, t1 or t2 for latissimus strength. At t2, the results were close to reveal a significant difference (U=71.50, Z=-1.919 p=0.055). Within-group comparisons showed a significant increase in IG over the study period (t0 median 22.94, t2 median 30.01, Z=-3.238 p=0.001). Delta change of CG mean -1.41 kg. (CI: -3.04 – 0.22) compared to IG mean change 6.87 kg. (CI: 3.58 – 10.15).
Crunch
No intergroup difference was noted in baseline measurements (U=109.50 Z=-0.416 p=0.678). During study participation at t1 (U=48.00, Z=-2.490, p=0.013) and t2 (U=50.50, Z=-2.750, p=0.006), significant changes in favour of IG were observed. Additionally, within-group analysis revealed a near-significant increase from t0 to t2 in the IG (t0 median 17.25, t2 median 23.00, Z=-2.731, p=0.06). Delta change of CG mean -1.81 kg. (CI: -4.09 – 0.47) compared to IG mean change 5.21 kg. (CI: 1.09 – 9.34).
Back extension
No intergroup difference was noted in baseline measurements (U=112.00, Z=-0.316, p=0.752), t1 (U=61.00, Z=-1.922, p=0.055) and t2 (U=71.00, Z=-1.938, p=0.053). Within-group comparisons showed a significant increase within the IG from t0 to t2 (t0 median 31.54, t2 median 46.00, Z=-3296, p<0.001). Delta change of CG mean 3.40 kg. (CI: -3.21 – 10.02) compared to IG mean change 21.72 kg. (CI: 8.27 – 35.17).
Patient-related outcome measurement (PROM)
Table 3 presents patient-related outcomes, assessing quality of life and physical activity levels using validated questionnaires. A significant difference between groups emerged at t2 for constipation (U=69.000, Z=-2.132, p<0.033). Throughout the trial, fatigue notably reduced in the IG (Z=-2.199, p<0.028). According to the BSA questionnaire, a noteworthy difference in weekly exercise minutes favored IG at t2 (U=56.000, Z=-2.505, p<0.012).
Subgroup analysis within aPDAC (n=19: IG, 9; CG, 10) displayed a significant advantage for IG in the physical function domain of quality of life at t2 (U=23.500, Z=-1.761, p<0.017). In the aBTC subgroup, the exercise group showed notably reduced constipation symptoms at t2. For the highly cachectic subgroup with over 5% body weight loss in the last 6 months (n=11: IG, 5; CG, 6), the quality-of-life score favored IG (U=1.000, Z=-2.586, p<0.009), and fatigue was significantly lower in IG (U=3.000, Z=-2.196, p<0.030) than in the CG.
Discussion
P-move investigated the feasibility and effects of exercise in patients with advanced PDAC or BTC beyond first line systemic treatment. We conducted a randomized controlled trial comparing patients who received exercise with those who were advised to exercise with the aim of assesing the feasibility of exercise based on recruitment rate, drop-out rate, adherence to exercise and adverse events. Within the IG, no adverse events related to exercise were reported, and a combination of supervised and guided home-based exercise during second-line treatment is considered safe. Compared to other trials in advanced pancreatic and biliary tract cancer (aPBC), our recruitment rate was distinctly higher [30, 31]. One reason for this may be that the study was conducted in a single-center comprehensive cancer center with the highest standard of care within a metropolitan area, which offers a shorter distance to the study centre, allowing more patients to participate without significantly increasing travel times. The drop-out rate was high as expected and comparable to other trials with advanced cancer patients [32, 33]. Patients demonstrated high adherence to both supervised and home-based training. Overall, P-move demonstrates the safety of exercise in this highly burdened patient population. We clearly demonstrate that even beyond first-line treatment exercise can affect physical function and improve patients’ well-being.
Clinical Implications
Based on recent exercise trials [30, 34] in advanced cancers, we expected exercise to provide physical and psychosocial benefits. Given the poor prognosis of PDAC and BTC, as assessed in our study, the treatment's emphasis lies in enhancing or maintaining quality of life, alleviating symptoms, and extending survival time. A general strength of our trial was the clinically embedded and feasible way of delivering (combination of supervised and home-based training) exercise intervention to patients under palliative systemic treatment without being too time consuming. We were able to integrate exercise within the current ambulant treatment system. Mostly, we offered the supervised exercise sessions before receiving anticancer treatment, or patients could decide which day would fit the best. Our results show that exercise can ensure and enhance patient mobility. These results, considering the frequent weight loss and loss of muscle mass during the trajectory of disease are an important signal that physical ability can be maintained or even improved by systematic and regular supervised exercise. These findings are important because cancer cachexia and sarcopenia are common and clinically significant challenges in aPBC, affecting physical functions, quality of life and reducing survival time. Pancreatic cancer patients are known to have physical function levels in the upper and lower body below the health reference values [35]. In particular, the functionality of the lower extremities enables patients to participate actively in social life and to cope optimally with regular everyday life. A qualitative study of advanced tumor patients with cachexia showed that patients want to take advantage of the positive potential of exercise and want to be as fit as possible during their therapy [36]. In our study we recognized that patients receiving exercise had fewer adverse events related to anti-tumor therapy. These findings support the hypothesis of an additional positive effect of exercise. A recent study showed that patients with advanced cancer who exercised during chemotherapy had significantly fewer chemotherapy dose delays, dose reductions [37, 38] or thrombocytopenia [39].
Despite the non-significant difference between groups in most patient-related outcome domains, we demonstrated a stabilization and slight increase in quality of life over 8 weeks of exercise in the intervention group. The EORTC-C30 demonstrates a general trend, with functional scales stabilizing or increasing slightly over the course of the trial and symptom scales decreasing in IG. Our findings gain increased significance based on the physical function scale as a recommended prognostic factor in PDAC [40]. Despite the same development/trend in the CG, the changes are considerably smaller. Subjective physical performance in our control group worsened over the course of study participation incorporate importance of exercise. Here, the change in chemotherapy plus the additional care aspect could be decisive. Quality of Life and physical function slightly decrease during study participation in CG compared to an increase in IG. One of the most common symptoms at the palliative cancer stage is fatigue, which affects almost 80% of all patients with advanced cancer [8]. From t0 to t2, our IG experienced significantly less fatigue. These findings are similar to those of Yeo et al. [41] during adjuvant treatment and highlight the beneficial aspect of exercise in palliative treatment. Based on our survey of patients' physical activity, the IG showed a significantly higher level of sports activity, which has a particular impact on fatigue and underlies the significant difference within group [42, 43].
Given our baseline blood sampling and the noteworthy differences between study completers and drop-outs, future investigations might benefit from using blood sampling as a stratification method during randomization. This approach could help balance dropouts across groups. Given NLR's established prognostic significance in both BTC [44, 45] and PDAC [46], along with its inclusion in routine clinical blood tests, it's prudent to consider it as a potential factor in randomization procedures.
Limitations
Even though our results demonstrate a benefit of exercise beyond first-line palliative treatment, our small sample size limits its generalizability. Based on the feasibility character of P-move, we did not power the study based on our secondary outcome; therefore, the results should be seen as providing a hypothesis to be tested in another upcoming larger multicentre randomized controlled trial. In P-move, we included 2 tumor types. Despite the limited number of cases, no distinct disparities in feasibility or exercise effects between these entities were identified. Notably, both tumor types exhibited similar responses to exercise. By demonstrating the benefits of exercise in cachectic patients, our study suggests a need for future research concentrating on those with tumor cachexia. Despite the small sample size, significant differences were observed, underscoring the potential impact. A major limitation is the impact of tumor therapy response on our cohort, which significantly influences psychological and physical aspects of patient-reported outcome measures (PROMs), though this variable remains uncontrolled. The method of measuring adherence to home-based training could not be fully tested by solely interviewing patients. Future studies should use validated tools to measure patient activity at home, such as accelerometers, devices such as smartwatches or pedometers, to control for reporting bias.
References
Halbrook CJ, Lyssiotis CA, di Magliano MP, Maitra A (2023) Pancreatic cancer: advances and challenges. Cell 186:1729–1754. https://doi.org/10.1016/j.cell.2023.02.014
Park W, Chawla A, O’Reilly EM (2021) Pancreatic cancer: a review. Jama 326:851–862. https://doi.org/10.1001/jama.2021.13027
Vogel A, Bridgewater J, Edeline J, Kelley R, Klümpen H, Malka D, Primrose J, Rimassa L, Stenzinger A, Valle JW (2023) Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 34:127–140. https://doi.org/10.1016/j.annonc.2022.10.506
Scott AJ, Sharman R, Shroff RT (2022) Precision medicine in biliary tract cancer. J Clin Oncol 40:2716–2734. https://doi.org/10.1200/JCO.21.02576
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281. https://doi.org/10.1056/NEJMoa0908721
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825. https://doi.org/10.1056/NEJMoa1011923
Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A (2007) Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage 34:94–104. https://doi.org/10.1016/j.jpainsymman.2006.10.015
Butt Z, Rosenbloom SK, Abernethy AP, Beaumont JL, Paul D, Hampton D, Jacobsen PB, Syrjala KL, Von Roenn JH, Cella D (2008) Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. J Natl Compr Canc Netw 6:448–455. https://doi.org/10.6004/jnccn.2008.0036
Wallengren O, Iresjö BM, Lundholm K, Bosaeus I (2015) Loss of muscle mass in the end of life in patients with advanced cancer. Support Care Cancer 23:79–86. https://doi.org/10.1007/s00520-014-2332-y
Moffat GT, Epstein AS, O'Reilly EM (2019) Pancreatic cancer-a disease in need: optimizing and integrating supportive care. Cancer 125:3927–3935. https://doi.org/10.1002/cncr.32423
Kalantar-Zadeh K, Rhee C, Sim JJ, Stenvinkel P, Anker SD, Kovesdy CP (2013) Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopenia Muscle 4:89–94. https://doi.org/10.1007/s13539-013-0111-0
Roeland EJ, Bohlke K, Baracos VE, Bruera E, Del Fabbro E, Dixon S, Fallon M, Herrstedt J, Lau H, Platek M, Rugo HS, Schnipper HH, Smith TJ, Tan W, Loprinzi CL (2020) Management of cancer cachexia: ASCO guideline. J Clin Oncol 38:2438–2453. https://doi.org/10.1200/jco.20.00611
Mattox TW (2017) Cancer cachexia: cause, diagnosis, and treatment. Nutr Clin Pract 32:599–606. https://doi.org/10.1177/0884533617722986
Mueller TC, Burmeister MA, Bachmann J, Martignoni ME (2014) Cachexia and pancreatic cancer: are there treatment options? World J Gastroenterol 20:9361–9373. https://doi.org/10.3748/wjg.v20.i28.9361
Ozola Zalite I, Zykus R, Francisco Gonzalez M, Saygili F, Pukitis A, Gaujoux S, Charnley RM, Lyadov V (2015) Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: a systematic review. Pancreatology 15:19–24. https://doi.org/10.1016/j.pan.2014.11.006
Bachmann J, Heiligensetzer M, Krakowski-Roosen H, Büchler MW, Friess H, Martignoni ME (2008) Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg 12:1193–1201. https://doi.org/10.1007/s11605-008-0505-z
Hilfiker R, Meichtry A, Eicher M, Nilsson Balfe L, Knols RH, Verra ML, Taeymans J (2018) Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med 52:651–658. https://doi.org/10.1136/bjsports-2016-096422
Belloni S, Arrigoni C, Caruso R (2021) Effects from physical exercise on reduced cancer-related fatigue: a systematic review of systematic reviews and meta-analysis. Acta Oncol 60:1678–1687. https://doi.org/10.1080/0284186X.2021.1962543
Misiąg W, Piszczyk A, Szymańska-Chabowska A, Chabowski M (2022) Physical activity and cancer care—a review. Cancers (Basel):14. https://doi.org/10.3390/cancers14174154
Grande AJ, Silva V, Sawaris Neto L, Teixeira Basmage JP, Peccin MS, Maddocks M (2021) Exercise for cancer cachexia in adults. Cochrane Database Syst Rev 3:Cd010804. https://doi.org/10.1002/14651858.CD010804.pub3
De Lazzari N, Niels T, Tewes M, Götte M (2021) A systematic review of the safety, feasibility and benefits of exercise for patients with advanced cancer. Cancers 13(17):4478. https://doi.org/10.3390/cancers13174478
Toohey K, Chapman M, Rushby AM, Urban K, Ingham G, Singh B (2023) The effects of physical exercise in the palliative care phase for people with advanced cancer: a systematic review with meta-analysis. J Cancer Surviv 17:399–415. https://doi.org/10.1007/s11764-021-01153-0
O'Connor D, Brown M, Eatock M, Turkington RC, Prue G (2021) Exercise efficacy and prescription during treatment for pancreatic ductal adenocarcinoma: a systematic review. BMC Cancer 21:43. https://doi.org/10.1186/s12885-020-07733-0
Brzycki M (1993) Strength testing—predicting a one-rep max from reps-to-fatigue. J Phys Educ Recreat Dance 64:88–90. https://doi.org/10.1080/07303084.1993.10606684
Hayes SC, Newton RU, Spence RR, Galvão DA (2019) The Exercise and Sports Science Australia position statement: Exercise medicine in cancer management. J Sci Med Sport 22:1175–1199. https://doi.org/10.1016/j.jsams.2019.05.003
Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, Zucker DS, Matthews CE, Ligibel JA, Gerber LH, Morris GS, Patel AV, Hue TF, Perna FM, Schmitz KH (2019) Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 51:2375–2390. https://doi.org/10.1249/mss.0000000000002116
Groenvold M, Klee MC, Sprangers MA, Aaronson NK (1997) Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J Clin Epidemiol 50:441–450. https://doi.org/10.1016/s0895-4356(96)00428-3
Fuchs R, Klaperski S, Gerber M, Seelig H (2015) Messung der Bewegungs-und Sportaktivität mit dem BSA-Fragebogen. Zeitschrift für Gesundheitspsychologie. https://doi.org/10.1026/0943-8149/a000137
Kieser M, Wassmer G (1996) On the use of the upper confidence limit for the variance from a pilot sample for sample size determination. Biom J 38:941–949. https://doi.org/10.1002/BIMJ.4710380806
Steindorf K, Clauss D, Tjaden C, Hackert T, Herbolsheimer F, Bruckner T, Schneider L, Ulrich CM, Wiskemann J (2019) Quality of life, fatigue, and sleep problems in pancreatic cancer patients: a randomized trial on the effects of exercise. Dtsch Arztebl Int 116:471–478. https://doi.org/10.3238/arztebl.2019.0471
Solheim TS, Laird BJA, Balstad TR, Stene GB, Bye A, Johns N, Pettersen CH, Fallon M, Fayers P, Fearon K, Kaasa S (2017) A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle 8:778–788. https://doi.org/10.1002/jcsm.12201
Henke CC, Cabri J, Fricke L, Pankow W, Kandilakis G, Feyer PC, de Wit M (2014) Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support Care Cancer 22:95–101. https://doi.org/10.1007/s00520-013-1925-1
Oldervoll LM, Loge JH, Lydersen S, Paltiel H, Asp MB, Nygaard UV, Oredalen E, Frantzen TL, Lesteberg I, Amundsen L, Hjermstad MJ, Haugen DF, Paulsen Ø, Kaasa S (2011) Physical exercise for cancer patients with advanced disease: a randomized controlled trial. Oncologist 16:1649–1657. https://doi.org/10.1634/theoncologist.2011-0133
Mikkelsen MK, Lund CM, Vinther A, Tolver A, Johansen JS, Chen I, Ragle AM, Zerahn B, Engell-Noerregaard L, Larsen FO, Theile S, Nielsen DL, Jarden M (2022) Effects of a 12-week multimodal exercise intervention among older patients with advanced cancer: results from a randomized controlled trial. Oncologist 27:67–78. https://doi.org/10.1002/onco.13970
Clauss D, Tjaden C, Hackert T, Schneider L, Ulrich CM, Wiskemann J, Steindorf K (2017) Cardiorespiratory fitness and muscle strength in pancreatic cancer patients. Cancer 25:2797–2807. https://doi.org/10.1007/s00520-017-3694-8
Bland KA, Krishnasamy M, Parr EB, Mulder S, Martin P, van Loon LJC, Cormie P, Michael N, Zopf EM (2022) "I want to get myself as fit as I can and not die just yet" - perceptions of exercise in people with advanced cancer and cachexia: a qualitative study. BMC Palliat Care 21:75. https://doi.org/10.1186/s12904-022-00948-x
Wonders KY, Schmitz K, Harness J (2023) Dose delays, dose reductions, and relative total dose intensity in patients with advanced cancer who exercised during neoadjuvant chemotherapy treatment. Integr Cancer Ther 22:15347354231168368. https://doi.org/10.1177/15347354231168368
Thomsen SN, Lahart IM, Thomsen LM, Fridh MK, Larsen A, Mau-Sørensen M, Bolam KA, Fairman CM, Christensen JF, Simonsen C (2023) Harms of exercise training in patients with cancer undergoing systemic treatment: a systematic review and meta-analysis of published and unpublished controlled trials. EClinicalMedicine 59:101937. https://doi.org/10.1016/j.eclinm.2023.101937
Mijwel S, Bolam KA, Gerrevall J, Foukakis T, Wengström Y, Rundqvist H (2020) Effects of exercise on chemotherapy completion and hospitalization rates: the OptiTrain breast cancer trial. Oncologist 25:23–32. https://doi.org/10.1634/theoncologist.2019-0262
Ter Veer E, van Rijssen LB, Besselink MG, Mali RM, Berlin JD, Boeck S, Bonnetain F, Chau I, Conroy T, van Cutsem E (2018) Consensus statement on mandatory measurements in pancreatic cancer trials (COMM-PACT) for systemic treatment of unresectable disease. Lancet Oncol 19:e151–e160. https://doi.org/10.1016/S1470-2045(18)30098-6
Yeo TP, Burrell SA, Sauter PK, Kennedy EP, Lavu H, Leiby BE, Yeo CJ (2012) A progressive postresection walking program significantly improves fatigue and health-related quality of life in pancreas and periampullary cancer patients. J Am Coll Surg 214:463–475; discussion 475-467. https://doi.org/10.1016/j.jamcollsurg.2011.12.017
Kummer F, Catuogno S, Perseus JM, Bloch W, Baumann FT (2013) Relationship between cancer-related fatigue and physical activity in inpatient cancer rehabilitation. Anticancer Res 33:3415–3422
Belloni S, Arrigoni C, Baroni I, Conte G, Dellafiore F, Ghizzardi G, Magon A, Villa G, Caruso R (2023) Non-pharmacologic interventions for improving cancer-related fatigue (CRF): a systematic review of systematic reviews and pooled meta-analysis. Semin Oncol 50(1–2):49–59. https://doi.org/10.1053/j.seminoncol.2023.03.004
Liu D, Heij LR, Czigany Z, Dahl E, Dulk MD, Lang SA, Ulmer TF, Neumann UP, Bednarsch J (2022) The prognostic value of neutrophil-to-lymphocyte ratio in cholangiocarcinoma: a systematic review and meta-analysis. Sci Rep 12:12691. https://doi.org/10.1038/s41598-022-16727-w
Iwai N, Okuda T, Sakagami J, Harada T, Ohara T, Taniguchi M, Sakai H, Oka K, Hara T, Tsuji T, Komaki T, Kagawa K, Yasuda H, Naito Y, Itoh Y (2020) Neutrophil to lymphocyte ratio predicts prognosis in unresectable pancreatic cancer. Sci Rep 10:18758. https://doi.org/10.1038/s41598-020-75745-8
Markus M, Abendroth A, Noureddine R, Paul A, Breitenbuecher S, Virchow I, Schmid KW, Markus P, Schumacher B, Wiesweg M, Wendling J, Mende B, Siveke JT, Schuler M, Kasper S (2021) Combined systemic inflammation score (SIS) correlates with prognosis in patients with advanced pancreatic cancer receiving palliative chemotherapy. J Cancer Res Clin Oncol 147:579–591. https://doi.org/10.1007/s00432-020-03361-0
Acknowledgements
We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.We acknowledge support by the Westdeutsche Biobank Essen (WBE, University Hospital Essen, University of Duisburg-Essen, Essen, Germany.
Data and material availability
No datasets were generated or analysed during the current study.
Funding
Open Access funding enabled and organized by Projekt DEAL. J.T.S is supported by the German Cancer Consortium (DKTK) and by the German Federal Ministry of Education and Research (BMBF; 01KD2206A/SATURN3).
We received funding from the Förderverein Innere Klinik -Tumorfoschung e. V. and the Stiftung Universitätsmedizin Essen.
Author information
Authors and Affiliations
Contributions
Conceptualization: [M. Tewes, J.T. Siveke, M. Götte]; Methodology: [M. Tewes, J.T. Siveke, M. Götte]; Formal analysis and investigation: [N. De Lazzari], ; Writing - original draft preparation: [N. De Lazzari]; Writing - review and editing: [N. De Lazzari, M. Tewes, J.T. Siveke, M. Götte, S. Kasper, M. Schuler, M. Pogorzelski, E. Meier]; Funding acquisition: [M. Tewes, J.T. Siveke, M. Götte]; Resources: [M. Pogorzelski, E. Meier, J.T. Siveke; M. Schuler]; Supervision: [M. Tewes, J.T. Siveke, M. Götte].
Corresponding author
Ethics declarations
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not applicable.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting of this research.
Conflict of Interest
J.T.S. receives honoraria as consultant or for continuing medical education presentations from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Immunocore, MSD Sharp Dohme, Novartis, Roche/Genentech, and Servier. His institution receives research funding from Abalos Therapeutics, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eisbach Bio, and Roche/Genentech; he holds ownership in FAPI Holding (<3%); all outside the submitted work.
S.K. received honoraria from Merck Serono, MSD, Novartis, BMS, Amgen, Roche, Sanofi-Aventis, Servier, Incyte and Lilly; research funding from Merck Serono, Lilly, BMS, Roche.
The other authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Lazzari, N., Götte, M., Kasper, S. et al. P-move: a randomized control trial of exercise in patients with advanced pancreatic or biliary tract cancer (aPBC) receiving beyond first-line chemotherapy. Support Care Cancer 32, 437 (2024). https://doi.org/10.1007/s00520-024-08650-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08650-9