Abstract
Rationale
Unintentional weight loss and malnutrition are common among cancer patients. Malnutrition has been associated with impaired health-related quality of life, less well-tolerated chemotherapy regimens and shorter life duration. In Belgium there is a lack of epidemiological data on malnutrition in oncology patients at advanced stages of the disease.
Methods
Malnutrition assessment data was collected through a prospective, observational study in 328 patients who started a neoadjuvant anticancer therapy regimen or who started 1st, 2nd or 3rd line anticancer therapy for a metastatic cancer via 3 visits according to regular clinical practice (baseline visit (BV) maximum 4 weeks before start therapy, 1st Follow up visit (FUV1) ± 6 weeks after start therapy, FUV2 ± 4 months after start therapy). Malnutrition screening was evaluated using the Nutritional Risk Screening score 2002 (NRS-2002)and the diagnosis of malnutrition by the GLIM criteria. In addition, SARC-F questionnaire and Fearon criteria were used respectively to screen for sarcopenia and cachexia.
Results
Prevalence of malnutrition risk at BV was high: 54.5% of the patients had a NRS ≥ 3 (NRS 2002) and increased during the study period (FUV1: 73.2%, FUV2: 70.1%). Prevalence of malnutrition based on physician subjective assessment (PSA) remained stable over the study period but was much lower compared to NRS results (14.0%—16.5%). At BV, only 10% of the patients got a nutrition plan and 43.9% received ≤ 70% of nutritional needs, percentage increased during FU period (FUV1: 68.4%, FUV2: 67.6%). Prevalence of sarcopenia and cachexia were respectively 12.4% and 38.1% at BV and without significant variation during the study period, but higher than assessed by PSA (11.6% and 6.7% respectively). Figures were also higher compared to PSA. There were modifications in cancer treatment at FUV1 (25.2%) and at FUV2 (50.8%). The main reasons for these modifications at FUV1 were adverse events and tolerability. Patient reported daily questionnaires of food intake showed early nutritional deficits, preceding clinical signs of malnutrition, and therefore can be very useful in the ambulatory setting.
Conclusions
Prevalence of malnutrition and cachexia was high in advanced cancer patients and underestimated by physician assessment. Earlier and rigorous detection of nutritional deficit and adjusted nutritional intake could lead to improved clinical outcomes in cancer patients. Reporting of daily caloric intake by patients was also very helpful with regards to nutritional assessment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malnutrition in cancer patients is a common problem, with varied prevalence depending on several parameters such as the type and symptomatic burden of the cancer, the history and stage of the disease or comorbidities of the patient. Cancer itself causes anorexia and impaired metabolism, but also cancer treatments such as chemotherapy, targeted therapy and radiotherapy have side effects that contribute to weight loss. Additionally, the physiological changes associated with cancer can lead to malabsorption, diarrhoea, vomiting, etc., contributing to the unintentional weight loss [1]. The systemic inflammation syndrome and the subsequent increase in the production of acute phase proteins result in altered protein turnover, loss of fat and muscle mass, insulin resistance, and impaired glucose tolerance [2]. Sarcopenia occurs as a result of muscle mass depletion and is commonly observed among cancer patients. A systematic review performed in 2017 estimated a 39% prevalence of sarcopenia at cancer diagnosis and associated the presence of pre-therapeutic sarcopenia with increased postoperative complications, chemotherapy-induced toxicity and poor survival in cancer patients [3].
The reported prevalence of malnutrition in recent studies including European patients with cancer ranges from 36% to over 50% [1, 4,5,6,7]. One study also identified that more than one third of oncology patients with a significantly decreased oral food intake, did not receive any kind of dietary advice or any prescription of oral supplements, which may contribute to their malnutrition status [1].
ESPEN guidelines state to use a validated screening tool to identify malnutrition in oncological patients in an early stage for instance with the Nutritional Risk Screening (NRS) 2002 [8, 9]. It is the first step towards implementing nutritional interventions to support patients during their disease and to improve disease outcomes, such as survival and patients’ quality of life [8].
Although there is a broad consensus that early recognition of malnutrition is important, there is a lack of implementation in daily clinical practice.
The recent ESMO guidelines [2] recommend the use of the consensus scheme offered by the Global Leadership Initiative in Malnutrition (GLIM) that defines malnutrition by the presence of a positive malnutrition screening test, one phenotypical criterium and one of two etiological criteria [5, 10].
In Belgium there is a lack of epidemiological data on malnutrition in oncology patients and clinical practice concerning screening, assessment and treating malnutrition seems to be very different between several centers.
Our first objective was to prospectively assess malnutrition prevalence according to the NRS 2002 score in patients with confirmed diagnosis of cancer in the need of neoadjuvant anticancer therapy or confirmed diagnosis of metastatic cancer who initiate first, second or third line palliative anticancer therapy in Belgium.
In addition, we wanted to evaluate malnutrition prevalence according to a composite malnutrition score and physician subjective assessment (PSA), to evaluate nutritional practices and clinical outcomes in the defined patient population, and to estimate the percentage of patients with sarcopenia and cachexia. The clinical outcomes for patients receiving an anticancer treatment were also monitored.
Patients and methods
Study design
ONCOCARE is a non-interventional prospective multicentric study of solid tumor patients who initiate a neoadjuvant anticancer therapy regimen or who initiate first, second or third line anticancer therapy for a metastatic cancer with palliative purposes.
The Ethics committee of the Antwerp University Hospital approved the study protocol in January 2019 (Belgian Registry nr: B300201837804).
Patient population
All participants were adult (> 18 years) starting an active oncologic treatment for a confirmed diagnosis of a solid tumor, locally advanced or at metastatic stage. Patient receiving radiotherapy or brachytherapy alone were excluded. All patients provided signed informed consent.
Objectives and outcomes
The primary study objectives were to assess malnutrition prevalence according to the NRS 2002 score [11] in patients with confirmed diagnosis of cancer in the need of neoadjuvant or metastatic anticancer therapy in Belgium, and to evaluate the diagnosis of malnutrition by according to a composite malnutrition score (GLIM criteria) [10] and physician subjective assessment (PSA), and to estimate the percentage of patients with a risk of sarcopenia and cachexia.
The secondary objective was to evaluate real-world nutritional practices and clinical outcomes in this defined patient population.
Method
Potentially eligible patients were invited to participate in the study at the baseline visit (BV), which occurred a maximum 4 weeks before initiation of the anticancer therapy regimen.
Once the patient was included, data at BV was collected by the oncologist with sociodemographic data and patient characteristics, cancer related clinical data including the type of oncologic treatment and the nutritional assessment. In addition, the patient collected data was using 2 questionnaires: one done only at baseline to estimate the mean kilocalories (kcal) intake during the three consecutive days before treatment (baseline questionnaire in appendix). A second questionnaire was performed daily to evaluate kcal intake and symptoms related to nutrition and anticancer therapy. A mean of kcal based on the daily questionnaire of the first week after inclusion was used for baseline. (Daily questionnaire in appendix).
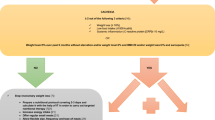
According to the clinical practice in Belgium, a follow-up visit 1 (FUV1) after 6 weeks (± 2 weeks) and a follow-up visit 2 (FUV2) after 16 weeks (± 2 weeks) was planned, as shown in Fig. 1.
During these FUV the same parameters as baseline were assessed by the oncologist and in addition also treatment related outcomes (treatment toxicity, treatment modification and unexpected hospitalization).
The malnutrition screening was evaluated according to current ESPEN guidelines using the Nutritional Risk Screening score 2002 (NRS-2002) [11] and the diagnosis of malnutrition by the GLIM criteria [10] as primary objective. For the diagnosis of malnutrition by the GLIM criteria it is clear that these cancer patients on chemotherapy/radiotherapy treatment for locally advanced or metastatic cancer fulfilled at least one etiology criterium (presence of inflammation and/or decreased food intake). As phenotypic criterium we used non-volitional weight loss and low BMI.
In our study we also assessed the use of Hand Grip Strength (HGS), as a functional assessment of decreased muscle mass, but this was not used in the GLIM criteria as the HGS is more a supportive measure, rather than a true assessor of decreased muscle mass. The HGS was measured as an indicator of the muscle strength of the upper extremities using a Jamar dynamometer on the dominant hand.
Patient’s nutritional needs were calculated based on ESPEN recommendations targeting 30 kcal/kg actual weight/day.
In addition, SARC-F questionnaire [12] and Fearon criteria [13] were used respectively to screen for sarcopenia and cachexia. The SARC-F questionnaire is a screening tool that can be rapidly implemented by clinicians to identify probable sarcopenic patients. The questionnaire screens patients for self-reported signs suggestive of sarcopenia, which include deficiencies in strength, walking, rising from a chair, climbing stairs, and experiencing falls. Each of the self-reported parameters receives a minimum and maximum score of 0 and 2, respectively, with the greatest maximum SARC-F score being 10. The Fearon criteria comprises a combination of weight loss and/or body mass index (BMI), and skeletal muscle index (determined by CT-imaging) for the diagnosis of cancer cachexia, in which an accurate estimate of weight loss is indispensable. In our study we did not use CT-imaging as it describes real-world observational epidemiological work.
The secondary objective was to evaluate the real-world nutritional practice and clinical outcome in this patient population in oncology wards in Belgium.
Statistical analyses
The sample size required for the study was calculated to assess the primary objective, to estimate the prevalence of malnutrition in patients receiving anticancer therapy based on NRS-2002. According to recent large European studies [1, 5,6,7, 13], the prevalence of malnutrition in the cancer population was estimated between 36 and 52%. A prevalence of 50% malnutrition in cancer patients has been used as an approximation for sample size calculation, corresponding to the prevalence requiring the highest sample size. To estimate a prevalence of 50% with a precision of 5%, corresponding to a 95% confidence interval between 45 and 55%, a sample of 385 valid patients is required. This sample was sufficient to assess the prevalence of malnutrition at baseline.
All data analyses were performed using SAS statistics software (SAS Institute Inc., Cary, NC) and in a manner consistent with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines and applicable sections of the Consolidated Standards of Reporting Trials (CONSORT) guidelines. No imputation of missing data was conducted. Patients for whom nutritional intake data collected through the patient diary was not available for > 7 consecutive calendar days, were considered as a drop out. For these patients, data for analysis was only considered until the date of the last available full nutritional intake data.
Univariate analysis was used to assess the relationship between malnutrition status and clinical outcomes or nutritional practice parameters. The statistical significance of the differences in the prevalence of malnutrition was assessed using the Chi-Square test. A logistical multivariate analysis was conducted to identify nutritional practices related to malnutrition, adjusting for potential confounders. Since all statistical tests were only exploratory, the type 1 error will not be adjusted for multiple testing.
Results
Patient characteristics
328 patients were included in the study. Table 1 shows the sociodemographic and clinical data of the patients at BV. 298 (90.9%) patients had a FUV1 (6 weeks ± 2 weeks after starting oncologic treatment). 259 patients (86.3%) completed a FUV2 (4 months after starting oncologic treatment). The majority of the patients had stage IV staging at the primary malignancy (75.3%, n = 247), stage II and III counted for respectively 11.3% and 10.3% of the included sample. Thirty seven percent (n = 91) of the patients had 1 metastatic site at first visit, 30.1% (n = 74) had 2 metastatic sites, and 32.9% (n = 81) of the patients had 3 or more metastatic sites. Seventy percent of patients (n = 229) received 1st line treatment at BV, 18% received 2nd line treatment and about 12% (11.9%, n = 39) received 3rd line treatment. At BV, the majority of the patients did belong to the category ECOG-PS 0 (48.2%, n = 158) and 1 (47.3%, n = 155). The patients belonging to category ECOG-PS 0 declined at FUV1 (6 weeks) to 39.4% (n = 117) and further declined at FUV2 (4 months) to 31.8% (N = 82).
Prevalence of (the risk of) malnutrition
Prevalence of the risk of malnutrition at BV, according to NRS 2002, was high: 54.5% of the patients (171 patients over a valid sample of 314) had a NRS > 3, meaning these patients were nutritionally at risk. This prevalence of risk of malnutrition increased after baseline: 73.2% of the patients had a NRS ≥ 3 at FUV1 and 70.1% of the patients at FUV2 were nutritionally at risk (NRS ≥ 3). Based on the GLIM score, 26.3% of the patients had malnutrition at BV with data only available for 224 out of 328 patients included in study sample. The prevalence of malnutrition increased over the study period (53.8% at FUV1, and 61.1% at FUV2). Evolution of GLIM scores during the study period must be interpreted with caution due to the low number of patients having complete information. Considering that a combination of different parameters (weight, height, nutritional need, malabsorption due to gastrointestinal disease and CRP) contribute to the GLIM score, the score could not be calculated if one of these parameters was not available. Prevalence of malnutrition at each visit was underestimated by the physician versus NRS 2002 and GLIM score. Table 2 shows the prevalence of (the risk of) malnutrition according to the NRS 2002 score, the GLIM score and according to the subjective assessment of the physician at each visit.
Nutritional assessment and intervention
Mean caloric intake decreased from 24.8 kcal/kg BW/day at BV to 17.8 kcal/kg BW/day at FUV1 and 17.4 kcal/kg BW/day at FUV2. Table 2 shows the nutritional intake, target, and balance at each visit. Other nutritional practices are presented in Table 3. At BV, only 10% (9.8%, n = 32) of the patients had a nutritional plan, and only 3 patients (0.9%) had a prescription of clinical nutrition (enteral and parenteral nutrition). More than half of the patients had consultation visits to the dietitian or nutritionist between the study visits (52.2% of the patients had consultation visits between BV and FUV1 and 53.5% of the patients had consultation visits between FUV1 and FUV2). These patients visiting a dietitian or nutritionist had on average 2.5 consultation visits between BV and FUV1 and between FUV1 and FUV2. The prescription of clinical nutrition increased slightly over the study period (FUV1: 2.0%, FUV2: 4.2%).
Clinical outcomes
The prevalence of sarcopenia and cachexia according to respectively SARC-F questionnaire and Fearon criteria, compared with the PSA, is presented in Table 2. For sarcopenia there is a good correlation between SARC-F questionnaire and PSA, the difference is more important for cachexia with an underestimation by the PSA compared to the Fearon criteria. A univariable analysis was done to assess the relationship between nutritional status at baseline and clinical outcome at follow up visit at 6 weeks (results presented in Table 3). Patients at risk of malnutrition at baseline show a higher frequency of sarcopenia assessed by PSA (6.6% vs 6.6%, p = 0.017), higher frequency of cachexia according to Fearon criteria (50.0% vs 19.7%, p < 0.001) and according to PSA (12.% vs 1.5%, p = 0.004), higher unexpected hospitalization rate (21.1% vs 10.9%, p = 0.027), and higher mortality rate (7.6% vs 2.1%, p = 0.027) at 6 weeks follow up visit. Similar results were noticed at 4 months follow up visit (data not shown). Multivariable analysis for the presence of cachexia and patient survival was not performed due to low number of patients with each of these conditions.
Table 4 presents the drug modifications and unexpected hospitalizations that occurred at FUV1 and 2. This data showed an increase of drug modification between BV & FUV1 (25% mainly with dose reduction and change in drug regimen), and between FUV1 & 2 (for 50,8% of patients mainly with drug regimen changes at 59,2%). About 95% (94.2%) of the patients presented symptoms and/or toxicities at FUV1. One hundred seventy-nine patients ( 84.8%) experienced fatigue/asthenia, 62.1% had quick satiety, 56.4% had constipation, 55.5% had nausea, half (50.2%) had diarrhoea and 46.9% of the patients had taste changes. Mucositis occurred in 25.6% of the patients and 21.8% of the patients experienced vomiting. Between BV and FUV1, 16.4% of the patients (n = 49) had an unexpected hospitalization. Most important reason for these unexpected hospitalizations was adverse events (81.8%).
Discussion
Our multicenter “real-life” cohort study confirms that in Belgium, as elsewhere, malnutrition remains underdiagnosed in oncological patients [1, 4,5,6,7]. At baseline, already more than half of cancer patients, regardless of the line of treatment, were at risk of malnutrition using NRS-2002 and a quarter of patients were malnourished using GLIM criteria as assessment tool. Interestingly, this nutritional status is largely underestimated by the subjective assessment of the treating oncologist, reinforcing the need to use validated and existing tools. It’s noteworthy that tools like NRS-2002 and GLIM, originally validated in hospitalized patients, could also be used to screen and assess malnutrition in our ambulatory oncological day clinic. An early multidisciplinary approach including oncologists, surgeons, specialized nurses and onco-dietitians, adapting nutritional support may impact outcomes with longer overall survival, less treatment modifications or discontinuations, better treatment tolerability with less adverse events and maintenance of quality of life [8].
During the observation period, risk of malnutrition prevalence even increased up to 70% within the first 6 weeks after baseline, while malnutrition was already prevalent up to 50%, showing the need for nutritional support throughout treatment, thereby limiting the risk of cachexia, aligned with literature [14,15,16,17]. Indeed, this study could show that the mean caloric intake was already deficient at baseline, decreasing significantly further after 6 weeks. Apparently, only 10% of patients received a nutrition plan at BV, mainly diet enrichment combined with the use of oral nutritional supplements, as first step as recommended in ESPEN guidelines. The use of clinical nutrition was limited (around 1%), with tube feeding or supplementary parenteral feeding hardly being considered. The lack of early nutritional support explains the increase in malnutrition rate, in parallel with a decrease of oral intake between BV and FUV1. It is only later, after 6 weeks and thereafter, that a dietetic consultation is considered with a potential impact on the stabilization of malnutrition rate and nutritional intake changes between FUV1 & 2. This data reinforces the need for early intervention by a dietitian, before any treatment initiation, in order to reduce the nutritional deficits and provide proper nutritional support.
Of course, our study has the well-known drawbacks of other observational studies with some bias and limitations but provides information on current practices in Belgian oncologic centers. ONCOCARE was an observational study, using NRS-2002 and the GLIM score as tools to detect risk of malnutrition and to assess malnutrition. Although the NRS-2002 is not a diagnostic tool, it was much more precise in detecting the risk of malnutrition rather than the PSA. Moreover, due to insufficient data for this observational study of real-world practices, no hard conclusion on the GLIM criteria could be made at this stage, as there were no data on muscle mass. In this study we had to limit the phenotypic GLIMS criteria to weight loss and low BMI. Apparently, the GLIM criteria, as a composite score, seems difficult to realize in daily practice. Nevertheless, the strength of this study is that this multicenter study, largest prospective study in Belgium evaluating the prevalence of malnutrition in cancer patients, as “real-world” data, confirming the findings of other European studies [5,6,7, 18] and providing an overview of the current clinical nutrition practices for advanced cancer patient treated with an active oncologic therapy. The completed patient daily questionnaire reporting outcomes also added interesting additional information to this study.
There is a significant difference between the prevalence of malnutrition using the screening tools (NRS 2002) and the physician subjective assessment. These results suggest a low detection of oncology patients at risk of malnutrition in terms of clinical practice in Belgium with a delay in starting an effective nutritional support and potentially reducing the negative impact on outcomes. As the physician’s judgement led to underestimation of malnutrition compared to existing tools, there is need for a multidisciplinary team effort to assess the nutritional status as early as possible after the diagnosis of cancer, before starting any treatment and during the treatment trajectory. There is probably a need to rethink the way of resources are allocated between in-hospital and ambulatory setting. In addition, an initiative to involve the patient with an active role in his/her oncological journey seems to be an attractive way to explore. Only then an impact can be expected on patient outcome, treatment modifications and maintenance of quality of life.
Indeed, a real added value to this study was the completed patient daily questionnaires of food intake as a patient reported outcome measurement (PROM) [daily questionnaire in appendix] evaluating nutritional deficits. These deficits, already present at-large during baseline visit, increase significantly in the first 6 weeks, thereby preceding the detection of malnutrition and/or sarcopenia by the existing tools like the NRS-2002, GLIM criteria or the Fearon cachexia criteria. PROMS have already demonstrated clinical efficacy in patients receiving anticancer treatment, however nutritional parameters are currently not included in the cross-cutting set of symptoms (e.g. pain, nausea, vomiting, constipation, diarrhea, dyspnea, insomnia, depression and physical function) as proposed by the ESMO guidelines [19]. If these PROMs can be picked up earlier and regularly by the multidisciplinary team, an early and adaptive intervention could be possible without waiting the scheduled follow-up visit. This could result in more patient empowerment increasing their motivation with an active role and offering a better quality of life with an adequate nutritional support.
Conclusion
Detecting nutritional deficits early, using PROMs and the existing tools of screening and assessment of malnutrition, and adjusted nutritional intake at initiation of any treatment, could lead to better clinical outcomes in cancer patients. The potential of PROMs in ambulatory oncological setting and nutrition support merits furthers prospective investigations.
Data Availability
Data are available on request, as they are stored on the repository of IQVIA (https://www.iqvia.com concerning IQVIA ref SFDC1208380) which can accessed on motivated request.
References
Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F (2014) Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr 38(2):196–204
Arends J, Strasser F, Gonella S, Solheim TS, Madeddu C, Ravasco P et al (2021) Cancer Cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 6(3):10092
Pamoukdjian et al (2017) Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin Nutr 37(4):1101–1113
Marshall et al (2019) Prevalence of manutrition and impacto n clinical outcomes in cancer services : a comparison of two timepoints. Clin Nutr 38(2):644–651
Gyan E, Raynard B, Durand JP, Lacau Saint Guily J, Gouy S, Movschin ML, NutriCancer2012 Investigator Group et al (2017) Malnutrition in patients with cancer. JPEN J Parenter Enteral Nutr 42(1):255–260
Muscaritoli M, Lucia S, Farcomeni A et al (2017) Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget 8(45):79884–79896
Planas M, Alvarez-Hernandez J, Leon-Sanz M, Celaya-Perez S, Araujo K (2016) Garcia de Lorenzo A, PREDyCES® researchers Prevalence of hospital malnutrition in cancer patients: a sub-analysis of the PREDyCES® study. Support Care Cancer 24:429–435
Muscaritoli et al (2021) ESPEN practical guidelines : clinical nutrition in cancer. Clin Nutr 40:2898–2913
Li et al (2019) Prognostic value of the nutritional risk screening 2002 scale in mestastatic gastric cancer : a large scale cohort study. J Cancer 10(1):112–119
Cederholm et al (2017) GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community; ESPEN endorsed recommendations. Clin Nutr 36(1):7–10
Kondrup J, Rasmussen H, Hamberg O, Stanga Z, Ad Hoc ESPEN Working Group (2003) Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 22(3):321–36
Mamstrom et al (2013) SARC-F : a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc 14(8):531–532
Fearon et al (2011) Definition and classification of cancer cachexia : an international consensus. Lancet Oncol 12(5):489–495
Vanhoutte et al (2016) Cachexia in cancer : what is the definition ? BMJ Open Gastroenterol 3:e000097
Ruggeri E et al (2020) Home artificial nutrition in palliative care cancer patients: Impact on survival and performance status. Clin Nutr 39(11):3346–3353
Roeland EJ, Bohlike K, Baracos VE et al (2020) Management of cancer cachexia: ASCO guideline. J Clin Oncol 38(21):2438–2453
Baldwin C (2015) The effectiveness of nutritional interventions in malnutrition and cachexia. Proc Nutr Soc 74(4):397–404
Meulemans A, Matthys C, Vangoitsenhoven R, Sabino J, Van Der Schuren B, Maertens P et al (2021) A multicenter propensity score matched analysis in 73,843 patients of an association of nutritional risk with mortality, length of stay and readmission rates. Am J Clin Nutr 114:1123–1130
Maio Di et al (2022) The role of patient-reported outcome measures in the continuum of cancer clinical care: ESMO Clinical Practice Guideline. Ann Oncol 33(9):878–892
Acknowledgements
The authors thank all coordinating investigators in the participating centers: CHU Ambroise Paré: Dr. Diaz Marie. Jan Yperman: Dr. Kurt Geldhof. ZNA Middelheim: Dr. Dirk Schrijvers. CSF Chimay: Dr. Oussama Hamdan. CHWAPI: Dr. Catherine Dophie. CHU Tivoli: Dr. Brigitte Vanderschueren. CHIREC: Dr. Corinne Grégoire. CHR Namur: Dr. Jean-Philippe Hermanne. The authors acknowledge Dr Hervé Chrostek (Fresenius Kabi CCO) and Louise Dewinter (Fresenius Kabi Belgium) for their thoughtful revision of the manuscript.
Funding
This observational study was sponsored by Fresenius Kabi.
Author information
Authors and Affiliations
Contributions
M.R., P.V., and D.Y. (1,2 and 12) contributed to the design of the study and wrote the first draft of the manuscript. The other authors included the most patients in the study and were involved in the discussion of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This non-interventional study was reviewed and approved by the ethics committee of the University hospital Antwerp (UZA) (No. B300201837804). This non-interventional study was carried out in line with the Declaration of Helsinki, as applicable.
Consent to participate
Written informed consent was obtained from all participating patients. All applicable data protection regulations were followed to ensure patient confidentiality.
Consent to publish
Not applicable.
Competing interests
Dr. Marika Rasschaert, prof. Dirk Ysebaert and Dr. Pieter Vandecandelaere received consulting fees from Fresenius Kabi as member of the Advisory Board for this observational study. All authors received fees from Fresenius Kabi for participation in this observational study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rasschaert, M., Vandecandelaere, P., Marechal, S. et al. Malnutrition prevalence in cancer patients in Belgium: The ONCOCARE study. Support Care Cancer 32, 135 (2024). https://doi.org/10.1007/s00520-024-08324-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08324-6