Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is a treatment-limiting adverse effect of anticancer therapy that complicates the lifestyle of many cancer survivors. There is currently no gold-standard for the assessment or management of CIPN. Subsequently, understanding the underlying mechanisms that lead to the development of CIPN is essential for finding better pharmacological therapy. Therapy-induced senescence (TIS) is a form of senescence that is triggered in malignant and non-malignant cells in response to the exposure to chemotherapy. Recent evidence has also suggested that TIS develops in the dorsal root ganglia of rodent models of CIPN. Interestingly, several components of the senescent phenotype are commensurate with the currently established primary processes implicated in the pathogenesis of CIPN including mitochondrial dysfunction, oxidative stress, and neuroinflammation. In this article, we review the literature that supports the hypothesis that TIS could serve as a holistic mechanism leading to CIPN, and we propose the potential for investigating senotherapeutics as means to mitigate CIPN in cancer survivors.

Similar content being viewed by others
References
Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN (2017) Neuropathic pain. Nat Rev Dis Primers 317002. https://doi.org/10.1038/nrdp.2017.2
Seretny M, Currie GL, Sena ES et al (2014) Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 155:2461–2470. https://doi.org/10.1016/J.PAIN.2014.09.020
Flatters SJL, Dougherty PM, Colvin LA (2017) Clinical and preclinical perspectives on chemotherapy-induced peripheral neuropathy (CIPN): a narrative review. Br J Anaesth 119:737–749. https://doi.org/10.1093/BJA/AEX229
Colvin LA (2019) Chemotherapy-induced peripheral neuropathy: where are we now? Pain 160(Suppl):S1–S10. https://doi.org/10.1097/J.PAIN.0000000000001540
Bai Y-G, Gao G-X, Zhang H et al (2020) Prognostic value of tumor-infiltrating lymphocyte subtypes in residual tumors of patients with triple-negative breast cancer after neoadjuvant chemotherapy. Chin Med J (Engl) 133:552–560. https://doi.org/10.1097/CM9.0000000000000656
Von Delius S, Eckel F, Wagenpfeil S et al (2007) Carbamazepine for prevention of oxaliplatin-related neurotoxicity in patients with advanced colorectal cancer: final results of a randomised, controlled, multicenter phase II study. Invest New Drugs 25:173–180. https://doi.org/10.1007/S10637-006-9010-Y
Gandara DR, Nahhas WA, Adelson MD et al (1995) Randomized placebo-controlled multicenter evaluation of diethyldithiocarbamate for chemoprotection against cisplatin-induced toxicities. J Clin Oncol 13:490–496. https://doi.org/10.1200/JCO.1995.13.2.490
Leal AD, Qin R, Atherton PJ et al (2014) North Central Cancer Treatment Group/Alliance trial N08CA-the use of glutathione for prevention of paclitaxel/carboplatin-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled study. Cancer 120:1890–1897. https://doi.org/10.1002/CNCR.28654
Schloss JM, Colosimo M, Airey C et al (2017) A randomised, placebo-controlled trial assessing the efficacy of an oral B group vitamin in preventing the development of chemotherapy-induced peripheral neuropathy (CIPN). Support Care Cancer 25:195–204. https://doi.org/10.1007/S00520-016-3404-Y
Loprinzi CL, Lacchetti C, Bleeker J et al (2020) Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol 38:3325–3348. https://doi.org/10.1200/JCO.20.01399
Bae EH, Greenwald MK, Schwartz AG (2021) Chemotherapy-induced peripheral neuropathy: mechanisms and therapeutic avenues. Neurotherapeutics 18:2384–2396. https://doi.org/10.1007/S13311-021-01142-2
Nelke C, Schroeter CB, Pawlitzki M et al (2022) Cellular senescence in neuroinflammatory disease: new therapies for old cells? Trends Mol Med 28:850–863. https://doi.org/10.1016/J.MOLMED.2022.07.003
Martínez-Cué C, Rueda N (2020) Cellular senescence in neurodegenerative diseases. Front Cell Neurosci 14:16. https://doi.org/10.3389/FNCEL.2020.00016
Sharpless NE, Sherr CJ (2015) Forging a signature of in vivo senescence. Nat Rev Cancer 15:397–408. https://doi.org/10.1038/nrc3960
Gorgoulis V, Adams PD, Alimonti A et al (2019) Cellular senescence: defining a path forward. Cell 179:813–827. https://doi.org/10.1016/j.cell.2019.10.005
Wiley CD, Campisi J (2021) The metabolic roots of senescence: mechanisms and opportunities for intervention. Nat Metab 3:1290–1301. https://doi.org/10.1038/S42255-021-00483-8
Lee BY, Han JA, Im JS et al (2006) Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 5:187–195. https://doi.org/10.1111/j.1474-9726.2006.00199.x
Sasaki M, Kumazaki T, Takano H et al (2001) Senescent cells are resistant to death despite low Bcl-2 level. Mech Ageing Dev 122:1695–1706. https://doi.org/10.1016/S0047-6374(01)00281-0
Zhang R, Poustovoitov MV, Ye X et al (2005) Formation of macroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev Cell 8:19–30. https://doi.org/10.1016/j.devcel.2004.10.019
Hernandez-Segura A, de Jong TV, Melov S et al (2017) Unmasking transcriptional heterogeneity in senescent cells. Curr Biol 27:2652–2660. https://doi.org/10.1016/j.cub.2017.07.033
Basisty N, Kale A, Jeon OH et al (2020) A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol 18:e3000599. https://doi.org/10.1371/journal.pbio.3000599
Coppé J-P, Desprez P-Y, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118. https://doi.org/10.1146/annurev-pathol-121808-102144
Acosta JC, Banito A, Wuestefeld T et al (2013) A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15:978–990. https://doi.org/10.1038/ncb2784
Saleh T, Bloukh S, Carpenter VJ et al (2020) Therapy-induced senescence: an “Old” Friend Becomes the enemy. Cancers (Basel) 12:822. https://doi.org/10.3390/cancers12040822
Saleh T, Alhesa A, Al-Balas M et al (2021) Expression of therapy-induced senescence markers in breast cancer samples upon incomplete response to neoadjuvant chemotherapy. Biosci Rep 41:BSR20210079. https://doi.org/10.1042/bsr20210079
El-Sadoni M, Al SS, Alhesa A et al (2023) A three-marker signature identifies senescence in human breast cancer exposed to neoadjuvant chemotherapy. Cancer Chemother Pharmacol 91:345–360. https://doi.org/10.1007/S00280-023-04523-W
Domen A, Deben C, De Pauw I et al (2022) Prognostic implications of cellular senescence in resected non-small cell lung cancer. Transl Lung Cancer Res 11:1526–1539
Domen A, Deben C, Verswyvel J et al (2022) Cellular senescence in cancer: clinical detection and prognostic implications. J Exp Clin Cancer Res 41:360. https://doi.org/10.1186/S13046-022-02555-3
Al Shboul S, El-Sadoni M, Alhesa A et al (2023) NOXA expression is downregulated in human breast cancer undergoing incomplete pathological response and senescence after neoadjuvant chemotherapy. Sci Rep 13:15903. https://doi.org/10.1038/s41598-023-42994-2
Saleh T, Bloukh S, Hasan M, Al Shboul S (2023) Therapy-induced senescence as a component of tumor biology: evidence from clinical cancer. Biochim Biophys Acta-Reviews Canceri 1878:188994. https://doi.org/10.1016/J.BBCAN.2023.188994
Demaria M, Leary MNO, Chang J et al (2017) Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov 7:165–177. https://doi.org/10.1158/2159-8290.CD-16-0241
Acklin S, Zhang M, Du W et al (2020) Depletion of senescent-like neuronal cells alleviates cisplatin-induced peripheral neuropathy in mice. Sci Rep 10:14170. https://doi.org/10.1038/s41598-020-71042-6
Calls A, Torres-Espin A, Navarro X et al (2021) Cisplatin-induced peripheral neuropathy is associated with neuronal senescence-like response. Neuro Oncol 23:88–99. https://doi.org/10.1093/NEUONC/NOAA151
Bousset L, Gil J (2022) Targeting senescence as an anticancer therapy. Mol Oncol 16:3855–3880. https://doi.org/10.1002/1878-0261.13312
Chaib S, Tchkonia T, Kirkland JL (2022) Cellular senescence and senolytics: the path to the clinic. Nat Med 28:1556–1568. https://doi.org/10.1038/S41591-022-01923-Y
Pachman DR, Qin R, Seisler D et al (2016) Comparison of oxaliplatin and paclitaxel-induced neuropathy (Alliance A151505). Support Care Cancer 24:5059–5068. https://doi.org/10.1007/S00520-016-3373-1
Kolb NA, Smith AG, Singleton JR et al (2016) The association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol 73:860–866. https://doi.org/10.1001/JAMANEUROL.2016.0383
Park SB, Goldstein D, Krishnan AV et al (2013) Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 63:419–437. https://doi.org/10.3322/CAAC.21204
Molassiotis A, Cheng HL, Lopez V, Au JSK, Chan A, Bandla A, Leung KT, Li YC, Wong KH, Suen LKP, Chan CW, Yorke J, Farrell C, Sundar R (2019) Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer 19(1):132. https://doi.org/10.1186/S12885-019-5302-4
Kamgar M, Greenwald MK, Assad H et al (2021) Prevalence and predictors of peripheral neuropathy after breast cancer treatment. Cancer Med 10:6666–6676. https://doi.org/10.1002/CAM4.4202
Kaiser K, Lyleroehr M, Shaunfield S et al (2020) Neuropathy experienced by colorectal cancer patients receiving oxaliplatin: a qualitative study to validate the functional assessment of cancer therapy/gynecologic oncology group-neurotoxicity scale. World J Gastrointest Oncol 12:205–218. https://doi.org/10.4251/WJGO.V12.I2.205
Mols F, Beijers T, Vreugdenhil G, Van De Poll-Franse L (2014) Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer 22:2261–2269. https://doi.org/10.1007/S00520-014-2255-7
Grisold W, Cavaletti G, Windebank AJ (2012) Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol 14(Suppl 4):iv45-54. https://doi.org/10.1093/NEUONC/NOS203
Smith EML, Pang H, Cirrincione C et al (2013) Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 309:1359–1367. https://doi.org/10.1001/JAMA.2013.2813
Olsen Y (2016) The CDC guideline on opioid prescribing: rising to the challenge. JAMA 315:1577–1579. https://doi.org/10.1001/JAMA.2016.1910
Avallone A, Bimonte S, Cardone C et al (2022) Pathophysiology and therapeutic perspectives for chemotherapy-induced peripheral neuropathy. Anticancer Res 42:4667–4678. https://doi.org/10.21873/ANTICANRES.15971
Zheng H, Xiao WH, Bennett GJ (2011) Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp Neurol 232:154–161. https://doi.org/10.1016/J.EXPNEUROL.2011.08.016
Carlson K, Ocean AJ (2011) Peripheral neuropathy with microtubule-targeting agents: occurrence and management approach. Clin Breast Cancer 11:73–81. https://doi.org/10.1016/J.CLBC.2011.03.006
Flatters SJL, Bennett GJ (2006) Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain 122:245–257. https://doi.org/10.1016/J.PAIN.2006.01.037
Chen YF, Chen LH, Yeh YM et al (2017) Minoxidil is a potential neuroprotective drug for paclitaxel-induced peripheral neuropathy. Sci Rep 7:45366. https://doi.org/10.1038/SREP45366
Jin HW, Flatters SJL, Xiao WH et al (2008) Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Exp Neurol 210:229–237. https://doi.org/10.1016/J.EXPNEUROL.2007.11.001
Nieto FR, Cendán CM, Cañizares FJ et al (2014) Genetic inactivation and pharmacological blockade of sigma-1 receptors prevent paclitaxel-induced sensory-nerve mitochondrial abnormalities and neuropathic pain in mice. Mol Pain 10:11. https://doi.org/10.1186/1744-8069-10-11
Xiao WH, Zheng H, Zheng FY et al (2011) Mitochondrial abnormality in sensory, but not motor, axons in paclitaxel-evoked painful peripheral neuropathy in the rat. Neuroscience 199:461–469. https://doi.org/10.1016/J.NEUROSCIENCE.2011.10.010
Imai S, Koyanagi M, Azimi Z et al (2017) Taxanes and platinum derivatives impair Schwann cells via distinct mechanisms. Sci Rep 7:5947. https://doi.org/10.1038/S41598-017-05784-1
Xiao WH, Zheng H, Bennett GJ (2012) Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience 203:194–206. https://doi.org/10.1016/J.NEUROSCIENCE.2011.12.023
Sharawy N, Rashed L, Youakim MF (2015) Evaluation of multi-neuroprotective effects of erythropoietin using cisplatin induced peripheral neurotoxicity model. Exp Toxicol Pathol 67:315–322. https://doi.org/10.1016/J.ETP.2015.02.003
Jamieson SMF, Liu J, Connor B, McKeage MJ (2005) Oxaliplatin causes selective atrophy of a subpopulation of dorsal root ganglion neurons without inducing cell loss. Cancer Chemother Pharmacol 56:391–399. https://doi.org/10.1007/S00280-004-0953-4
Zhang H, Dougherty PM (2014) Enhanced excitability of primary sensory neurons and altered gene expression of neuronal ion channels in dorsal root ganglion in paclitaxel-induced peripheral neuropathy. Anesthesiology 120:1463–1475. https://doi.org/10.1097/ALN.0000000000000176
Li Y, North RY, Rhines LD et al (2018) DRG voltage-gated sodium channel 1.7 is upregulated in paclitaxel-induced neuropathy in rats and in humans with neuropathic pain. J Neurosci 38:1124–1136. https://doi.org/10.1523/JNEUROSCI.0899-17.2017
Sittl R, Lampert A, Huth T et al (2012) Anticancer drug oxaliplatin induces acute cooling-aggravated neuropathy via sodium channel subtype Na(V)1.6-resurgent and persistent current. Proc Natl Acad Sci 109:6704–6709. https://doi.org/10.1073/PNAS.1118058109
Park SB, Goldstein D, Lin CSY et al (2009) Acute abnormalities of sensory nerve function associated with oxaliplatin-induced neurotoxicity. J Clin Oncol 27:1243–1249. https://doi.org/10.1200/JCO.2008.19.3425
Thibault K, Calvino B, Dubacq S et al (2012) Cortical effect of oxaliplatin associated with sustained neuropathic pain: exacerbation of cortical activity and down-regulation of potassium channel expression in somatosensory cortex. Pain 153:1636–1647. https://doi.org/10.1016/J.PAIN.2012.04.016
Descoeur J, Pereira V, Pizzoccaro A et al (2011) Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol Med 3:266–278. https://doi.org/10.1002/EMMM.201100134
Caterina MJ, Schumacher MA, Tominaga M et al (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824. https://doi.org/10.1038/39807
Li Y, Adamek P, Zhang H et al (2015) The cancer chemotherapeutic paclitaxel increases human and rodent sensory neuron responses to TRPV1 by activation of TLR4. J Neurosci 35:13487–13500. https://doi.org/10.1523/JNEUROSCI.1956-15.2015
Hara T, Chiba T, Abe K et al (2013) Effect of paclitaxel on transient receptor potential vanilloid 1 in rat dorsal root ganglion. Pain 154:882–889. https://doi.org/10.1016/J.PAIN.2013.02.023
Ta LE, Bieber AJ, Carlton SM et al (2010) Transient receptor potential vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol Pain 6:15. https://doi.org/10.1186/1744-8069-6-15
Nassini R, Gees M, Harrison S et al (2011) Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain 152:1621–1631. https://doi.org/10.1016/J.PAIN.2011.02.051
Zhao M, Isami K, Nakamura S et al (2012) Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol Pain 8:55. https://doi.org/10.1186/1744-8069-8-55
Trevisan G, Materazzi S, Fusi C et al (2013) Novel therapeutic strategy to prevent chemotherapy-induced persistent sensory neuropathy by TRPA1 blockade. Cancer Res 73:3120–3131. https://doi.org/10.1158/0008-5472.CAN-12-4370
Proudfoot CJ, Garry EM, Cottrell DF et al (2006) Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol 16:1591–1605. https://doi.org/10.1016/J.CUB.2006.07.061
Storey DJ, Colvin LA, Mackean MJ et al (2010) Reversal of dose-limiting carboplatin-induced peripheral neuropathy with TRPM8 activator, menthol, enables further effective chemotherapy delivery. J Pain Symptom Manage 39:e2-4. https://doi.org/10.1016/J.JPAINSYMMAN.2010.02.004
Cavaletti G, Cavalletti E, Oggioni N et al (2001) Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. J Peripher Nerv Syst 6:61–61. https://doi.org/10.1046/J.1529-8027.2001.01008-5.X
Melli G, Jack C, Lambrinos GL et al (2006) Erythropoietin protects sensory axons against paclitaxel-induced distal degeneration. Neurobiol Dis 24:525–530. https://doi.org/10.1016/J.NBD.2006.08.014
Siau C, Xiao W, Bennett GJ (2006) Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exp Neurol 201:507–514. https://doi.org/10.1016/J.EXPNEUROL.2006.05.007
Zhang H, Boyette-Davis JA, Kosturakis AK et al (2013) Induction of monocyte chemoattractant protein-1 (MCP-1) and its receptor CCR2 in primary sensory neurons contributes to paclitaxel-induced peripheral neuropathy. J pain 14:1031–1044. https://doi.org/10.1016/J.JPAIN.2013.03.012
Warwick RA, Hanani M (2013) The contribution of satellite glial cells to chemotherapy-induced neuropathic pain. Eur J Pain 17:571–580. https://doi.org/10.1002/J.1532-2149.2012.00219.X
González H, Pacheco R (2014) T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J Neuroinflammation 11:201. https://doi.org/10.1186/S12974-014-0201-8
Janes K, Wahlman C, Little JW et al (2015) Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav Immunitymmunity 44:91–99. https://doi.org/10.1016/J.BBI.2014.08.010
Jin X, Gereau RW IV (2006) Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci 26:246–255. https://doi.org/10.1523/JNEUROSCI.3858-05.2006
Sun JH, Yang B, Donnelly DF et al (2006) MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol 96:2189–2199. https://doi.org/10.1152/JN.00222.2006
Li YY, Li H, Liu ZL et al (2017) Activation of STAT3-mediated CXCL12 up-regulation in the dorsal root ganglion contributes to oxaliplatin-induced chronic pain. Mol Pain 13:1744806917747425. https://doi.org/10.1177/1744806917747425
Cho HS, Choi YI, Park SU et al (2023) Prevention of chemotherapy-induced peripheral neuropathy by inhibiting C-X-C motif chemokine receptor 2. Int J Mol Sci 24:1855. https://doi.org/10.3390/IJMS24031855
Park HJ, Stokes JA, Corr M, Yaksh TL (2014) Toll-like receptor signaling regulates cisplatin-induced mechanical allodynia in mice. Cancer Chemother Pharmacol 73:25–34. https://doi.org/10.1007/S00280-013-2304-9
Li C, Deng T, Shang Z et al (2018) Blocking TRPA1 and TNF-α signal improves bortezomib-induced neuropathic pain. Cell Physiol Biochem 51:2098–2110. https://doi.org/10.1159/000495828
Xu D, Zhao H, Gao H et al (2018) Participation of pro-inflammatory cytokines in neuropathic pain evoked by chemotherapeutic oxaliplatin via central GABAergic pathway. Mol Pain 14:1744806918783535. https://doi.org/10.1177/1744806918783535
Wang Ys, Li Yy, Cui W et al (2017) Melatonin attenuates pain hypersensitivity and decreases astrocyte-mediated spinal neuroinflammation in a rat model of oxaliplatin-induced pain. Inflammation 40:2052–2061. https://doi.org/10.1007/S10753-017-0645-Y
Li Y, Zhang H, Zhang H et al (2014) Toll-like receptor 4 signaling contributes to paclitaxel-induced peripheral neuropathy. J Pain 15:712–725. https://doi.org/10.1016/J.JPAIN.2014.04.001
Zhang H, Yoon SY, Zhang H, Dougherty PM (2012) Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of paclitaxel-induced painful neuropathy. J pain 13:293–303. https://doi.org/10.1016/J.JPAIN.2011.12.002
Krukowski K, Eijkelkamp N, Laumet G et al (2016) CD8+ T cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. J Neurosci 36:11074–11083. https://doi.org/10.1523/JNEUROSCI.3708-15.2016
Liu CC, Lu N, Cui Y et al (2010) Prevention of paclitaxel-induced allodynia by minocycline: effect on loss of peripheral nerve fibers and infiltration of macrophages in rats. Mol Pain 6:76. https://doi.org/10.1186/1744-8069-6-76
Salvemini D, Doyle T, Chen Z et al (2012) Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. J Neurosci 32:6149–6160. https://doi.org/10.1523/JNEUROSCI.6343-11.2012
Li Y, Kang J, Xu Y, Li N, Jiao Y, Wang C, Wang C, Wang G, Yu Y, Yuan J, Zhang L (2022) Artesunate alleviates paclitaxel-induced neuropathic pain in mice by decreasing metabotropic glutamate receptor 5 activity and neuroinflammation in primary sensory neurons. Front Mol Neurosci 15:902572. https://doi.org/10.3389/fnmol.2022.902572
Campisi J (1997) The biology of replicative senescence. Eur J Cancer 33:703–709. https://doi.org/10.1016/S0959-8049(96)00058-5
Campisi J, d’Adda di Fagagna F (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8:729–740. https://doi.org/10.1038/nrm2233
Fitsiou E, Soto-Gamez A, Demaria M (2022) Biological functions of therapy-induced senescence in cancer. Semin Cancer Biol 81:5–13. https://doi.org/10.1016/j.semcancer.2021.03.021
Serrano M, Lin AW, McCurrach ME et al (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16(INK4a). Cell 88:593–602. https://doi.org/10.1016/S0092-8674(00)81902-9
DeLuca VJ, Saleh T (2023) Insights into the role of senescence in tumor dormancy: mechanisms and applications. Cancer Metastasis Rev 42:19–35. https://doi.org/10.1007/S10555-023-10082-6
Saleh T, Gewirtz DA (2022) Considering therapy-induced senescence as a mechanism of tumour dormancy contributing to disease recurrence. Br J Cancer 126:1363–1365. https://doi.org/10.1038/S41416-022-01787-6
Milanovic M, Fan DNY, Belenki D et al (2018) Senescence-associated reprogramming promotes cancer stemness. Nature 553:96–100. https://doi.org/10.1038/nature25167
Qi Z, Zhang Y, Liu L et al (2012) Mesenchymal stem cells derived from different origins have unique sensitivities to different chemotherapeutic agents. Cell Biol Int 36:857–862. https://doi.org/10.1042/cbi20110637
Yosef R, Pilpel N, Papismadov N et al (2017) p21 maintains senescent cell viability under persistent DNA damage response by restraining JNK and caspase signaling. EMBO J 36:2280–2295. https://doi.org/10.15252/embj.201695553
Zhao H, Halicka HD, Traganos F et al (2010) New biomarkers probing depth of cell senescence assessed by laser scanning cytometry. Cytom Part A 77:999–1007. https://doi.org/10.1002/cyto.a.20983
Probin V, Wang Y, Bai A, Zhou D (2006) Busulfan selectively induces cellular senescence but not apoptosis in WI38 fibroblasts via a p53-independent but extracellular signal-regulated kinase-p38 mitogen-activated protein kinase-dependent mechanism. J Pharmacol Exp Ther 319:551–560. https://doi.org/10.1124/jpet.106.107771
Probin V, Wang Y, Zhou D (2007) Busulfan-induced senescence is dependent on ROS production upstream of the MAPK pathway. Free Radic Biol Med 42:1858–1865. https://doi.org/10.1016/j.freeradbiomed.2007.03.020
Cells MH, Meng A, Wang Y et al (2003) Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res 63:5414–5419
Marques L, Johnson AA, Stolzing A (2020) Doxorubicin generates senescent microglia that exhibit altered proteomes, higher levels of cytokine secretion, and a decreased ability to internalize amyloid β. Exp Cell Res 395:112203. https://doi.org/10.1016/J.YEXCR.2020.112203
Moruno-Manchon JF, Uzor NE, Kesler SR et al (2018) Peroxisomes contribute to oxidative stress in neurons during doxorubicin-based chemotherapy. Mol Cell Neurosci 86:65. https://doi.org/10.1016/J.MCN.2017.11.014
Piechota M, Sunderland P, Wysocka A et al (2016) Is senescence-associated β-galactosidase a marker of neuronal senescence? Oncotarget 7:81099–81109. https://doi.org/10.18632/ONCOTARGET.12752
Bojko A, Czarnecka-Herok J, Charzynska A et al (2019) Diversity of the senescence phenotype of cancer cells treated with chemotherapeutic agents. Cells 8:1501. https://doi.org/10.3390/cells8121501
Lérida-Viso A, Estepa-Fernández A, Morellá-Aucejo Á et al (2022) Pharmacological senolysis reduces doxorubicin-induced cardiotoxicity and improves cardiac function in mice. Pharmacol Res 183:106356. https://doi.org/10.1016/J.PHRS.2022.106356
Münz F, Lopez Perez R, Trinh T et al (2018) Human mesenchymal stem cells lose their functional properties after paclitaxel treatment. Sci Rep 8:312. https://doi.org/10.1038/S41598-017-18862-1
Ota H, Eto M, Ako J et al (2009) Sirolimus and everolimus induce endothelial cellular senescence via sirtuin 1 down-regulation: therapeutic implication of cilostazol after drug-eluting stent implantation. J Am Coll Cardiol 53:2298–2305. https://doi.org/10.1016/j.jacc.2009.01.072
Limbad C, Oron TR, Alimirah F et al (2020) Astrocyte senescence promotes glutamate toxicity in cortical neurons. PLoS One 15:e0227887. https://doi.org/10.1371/JOURNAL.PONE.0227887
Turnquist C, Beck JA, Horikawa I et al (2019) Radiation-induced astrocyte senescence is rescued by Δ133p53. Neuro Oncol 21:474–485. https://doi.org/10.1093/NEUONC/NOZ001
Jurk D, Wang C, Miwa S et al (2012) Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell 11:996–1004. https://doi.org/10.1111/J.1474-9726.2012.00870.X
Park JT, Lee YS, Cho KA, Park SC (2018) Adjustment of the lysosomal-mitochondrial axis for control of cellular senescence. Ageing Res Rev 47:176–182. https://doi.org/10.1016/j.arr.2018.08.003
Miwa S, Kashyap S, Chini E, von Zglinicki T (2022) Mitochondrial dysfunction in cell senescence and aging. J Clin Invest 132:e158447. https://doi.org/10.1172/JCI158447
Wiley CD, Velarde MC, Lecot P et al (2016) Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab 23:303–314. https://doi.org/10.1016/J.CMET.2015.11.011
Wiel C, Lallet-Daher H, Gitenay D et al (2014) Endoplasmic reticulum calcium release through ITPR2 channels leads to mitochondrial calcium accumulation and senescence. Nat Commun 5:3792. https://doi.org/10.1038/NCOMMS4792
Pradeepkiran JA, Baig J, Selman A, Reddy PH (2023) Mitochondria in aging and Alzheimer’s disease: focus on mitophagy. Neurosci. https://doi.org/10.1177/10738584221139761
Strickland M, Yacoubi-Loueslati B, Bouhaouala-Zahar B et al (2019) Relationships between ion channels, mitochondrial functions and inflammation in human aging. Front Physiol 10:158. https://doi.org/10.3389/FPHYS.2019.00158
Guerrero A, De Strooper B, Arancibia-Cárcamo IL (2021) Cellular senescence at the crossroads of inflammation and Alzheimer’s disease. Trends Neurosci 44:714–727. https://doi.org/10.1016/J.TINS.2021.06.007
Nicaise AM, Wagstaff LJ, Willis CM, Paisie C, Chandok H, Robson P, Fossati V, Williams A, Crocker SJ (2019) Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc Natl Acad Sci U S A 116(18):9030–9039. https://doi.org/10.1073/pnas.1818348116
Chambers ES, Vukmanovic-Stejic M, Shih BB et al (2021) Recruitment of inflammatory monocytes by senescent fibroblasts inhibits antigen-specific tissue immunity during human aging. Nat Aging 11(1):101–113. https://doi.org/10.1038/s43587-020-00010-6
Krizhanovsky V, Yon M, Dickins RA et al (2008) Senescence of activated stellate cells limits liver fibrosis. Cell 134:657–667. https://doi.org/10.1016/j.cell.2008.06.049
Sagiv A, Biran A, Yon M et al (2012) Granule exocytosis mediates immune surveillance of senescent cells. Oncogene 32:1971–1977. https://doi.org/10.1038/onc.2012.206
Ortiz-Montero P, Londoño-Vallejo A, Vernot JP (2017) Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun Signal 15:1–18. https://doi.org/10.1186/s12964-017-0172-3
Calvo-Rodríguez M, de la Fuente C, García-Durillo M et al (2017) Aging and amyloid β oligomers enhance TLR4 expression, LPS-induced Ca2+ responses, and neuron cell death in cultured rat hippocampal neurons. J Neuroinflammation 14:24. https://doi.org/10.1186/S12974-017-0802-0
Mitin N, Nyrop KA, Strum SL et al (2022) A biomarker of aging, p16, predicts peripheral neuropathy in women receiving adjuvant taxanes for breast cancer. NPJ Breast Cancer 8:103. https://doi.org/10.1038/S41523-022-00473-3
Liu Y, Sanoff HK, Cho H et al (2009) Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell 8:439–448. https://doi.org/10.1111/J.1474-9726.2009.00489.X
Üçeyler N, Kafke W, Riediger N et al (2010) Elevated proinflammatory cytokine expression in affected skin in small fiber neuropathy. Neurology 74:1806–1813. https://doi.org/10.1212/WNL.0B013E3181E0F7B3
Tonello R, Lee SH, Berta T (2019) Monoclonal antibody targeting the matrix metalloproteinase 9 prevents and reverses paclitaxel-induced peripheral neuropathy in mice. J Pain 20:515–527. https://doi.org/10.1016/J.JPAIN.2018.11.003
Du J, Cheng N, Deng Y et al (2023) Astrocyte senescence-like response related to peripheral nerve injury-induced neuropathic pain. Cell Mol Biol Lett 28:65. https://doi.org/10.1186/S11658-023-00474-5
Saleh T, Carpenter V, Tyutyunyk-Massey L et al (2020) Clearance of therapy-induced senescent tumor cells by the senolytic ABT-263 via interference with BCL-X L -BAX Interaction. Mol Oncol 14:1–16. https://doi.org/10.1002/1878-0261.12761
Zhu Y, Tchkonia T, Pirtskhalava T et al (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14:644–658. https://doi.org/10.1111/acel.12344
Myrianthopoulos V, Evangelou K, Vasileiou PVS, Cooks T, Vassilakopoulos TP, Pangalis GA, Kouloukoussa M, Kittas C, Georgakilas AG, Gorgoulis VG (2019) Senescence and senotherapeutics: a new field in cancer therapy. Pharmacol Ther 193:31–49. https://doi.org/10.1016/j.pharmthera.2018.08.006
Yang H, Chen C, Chen H et al (2020) Navitoclax (ABT263) reduces inflammation and promotes chondrogenic phenotype by clearing senescent osteoarthritic chondrocytes in osteoarthritis. Aging (Albany NY) 12:12750–12770. https://doi.org/10.18632/aging.103177
Carpenter VJ, Saleh T, Gewirtz DA (2021) Senolytics for cancer therapy: is all that glitters really gold? Cancers (Basel) 13:723. https://doi.org/10.3390/cancers13040723
Lee YR, Cho HM, Park EJ et al (2020) Metabolite profiling of rambutan (Nephelium lappaceum L.) seeds using UPLC-qTOF-MS/MS and senomorphic effects in aged human dermal fibroblasts. Nutrients 12:1430. https://doi.org/10.3390/NU12051430
Laberge R, Sun Y, Orjalo AV et al (2015) MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 17:1049–1061. https://doi.org/10.1038/ncb3195
Xu C, Shen WB, Albert Reece E et al (2021) Maternal diabetes induces senescence and neural tube defects sensitive to the senomorphic rapamycin. Sci Adv 7:eabf5089. https://doi.org/10.1126/SCIADV.ABF5089
Acar MB, Ayaz-Güner Ş, Gunaydin Z et al (2021) Proteomic and biological analysis of the effects of metformin senomorphics on the mesenchymal stromal cells. Front Bioeng Biotechnol 9:730813. https://doi.org/10.3389/FBIOE.2021.730813
Malaquin N, Vancayseele A, Gilbert S, Antenor-Habazac L, Olivier MA, Ait Ali Brahem Z, Saad F, Delouya G, Rodier F (2020) DNA damage- but not enzalutamide-induced senescence in prostate cancer promotes senolytic Bcl-xL inhibitor sensitivity. Cells 9(7):1593. https://doi.org/10.3390/cells9071593
Lim JS, Lee DY, Kim HS et al (2020) Identification of a novel senomorphic agent, avenanthramide C, via the suppression of the senescence-associated secretory phenotype. Mech Ageing Dev 192:111355. https://doi.org/10.1016/J.MAD.2020.111355
Zhang X, Dong Y, Li Wc et al (2021) Roxithromycin attenuates bleomycin-induced pulmonary fibrosis by targeting senescent cells. Acta Pharmacol Sin 42:2058–2068. https://doi.org/10.1038/S41401-021-00618-3
He Y, Yocum L, Alexander PG et al (2021) Urolithin A protects chondrocytes from mechanical overloading-induced injuries. Front Pharmacol 12:703847. https://doi.org/10.3389/FPHAR.2021.703847
Śmieszek A, Stręk Z, Kornicka K et al (2017) Antioxidant and anti-senescence effect of metformin on mouse olfactory ensheathing cells (mOECs) may be associated with increased brain-derived neurotrophic factor levels-an ex vivo study. Int J Mol Sci 18:872. https://doi.org/10.3390/IJMS18040872
Moiseeva O, Deschênes-Simard X, St-Germain E et al (2013) Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell 12:489–498. https://doi.org/10.1111/acel.12075
Le Pelletier L, Mantecon M, Gorwood J et al (2021) Metformin alleviates stress-induced cellular senescence of aging human adipose stromal cells and the ensuing adipocyte dysfunction. Elife 10:e62635. https://doi.org/10.7554/ELIFE.62635
Mao-Ying QL, Kavelaars A, Krukowski K et al (2014) The anti-diabetic drug metformin protects against chemotherapy-induced peripheral neuropathy in a mouse model. PLoS One 9:e100701. https://doi.org/10.1371/JOURNAL.PONE.0100701
Ludman T, Melemedjian OK (2019) Bortezomib and metformin opposingly regulate the expression of hypoxia-inducible factor alpha and the consequent development of chemotherapy-induced painful peripheral neuropathy. Mol Pain 15:1744806919850043. https://doi.org/10.1177/1744806919850043
Alotaibi M, Al-Aqil F, Alqahtani F, Alanazi M, Nadeem A, Ahmad SF, Lapresa R, Alharbi M, Alshammari A, Alotaibi M, Saleh T, Alrowis R (2022) Alleviation of cisplatin-induced neuropathic pain, neuronal apoptosis, and systemic inflammation in mice by rapamycin. Front Aging Neurosci 14:891593. https://doi.org/10.3389/FNAGI.2022.891593
Wang R, Yu Z, Sunchu B et al (2017) Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell 16:564–574. https://doi.org/10.1111/acel.12587
Asante CO, Wallace VC, Dickenson AH (2010) Mammalian target of rapamycin signaling in the spinal cord is required for neuronal plasticity and behavioral hypersensitivity associated with neuropathy in the rat. J Pain. https://doi.org/10.1016/j.jpain.2010.03.013
Wang X, Li X, Huang B, Ma S (2016) Blocking mammalian target of rapamycin (mTOR) improves neuropathic pain evoked by spinal cord injury. Transl Neurosci. https://doi.org/10.1515/tnsci-2016-0008
Uddin MS, Al Mamun A, Rahman MA et al (2020) Exploring the promise of flavonoids to combat neuropathic pain: from molecular mechanisms to therapeutic implications. Front Neurosci 9:478. https://doi.org/10.3389/FNINS.2020.00478
Schwingel TE, Klein CP, Nicoletti NF et al (2014) Effects of the compounds resveratrol, rutin, quercetin, and quercetin nanoemulsion on oxaliplatin-induced hepatotoxicity and neurotoxicity in mice. Naunyn Schmiedebergs Arch Pharmacol 387:837–848. https://doi.org/10.1007/S00210-014-0994-0
Zhao X, Wang C, Cui WG et al (2015) Fisetin exerts antihyperalgesic effect in a mouse model of neuropathic pain: engagement of spinal serotonergic system. Sci Rep 5:9043. https://doi.org/10.1038/SREP09043
Yousefzadeh MJ, Zhu Y, McGowan SJ et al (2018) Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36:18–28. https://doi.org/10.1016/j.ebiom.2018.09.015
Nabavi SF, Khan H, D’onofrio G et al (2018) Apigenin as neuroprotective agent: of mice and men. Pharmacol Res 128:359–365. https://doi.org/10.1016/J.PHRS.2017.10.008
Lim H, Park H, Kim HP (2015) Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. Biochem Pharmacol 96:337–348. https://doi.org/10.1016/J.BCP.2015.06.013
Valsecchi AE, Franchi S, Panerai AE et al (2008) Genistein, a natural phytoestrogen from soy, relieves neuropathic pain following chronic constriction sciatic nerve injury in mice: anti-inflammatory and antioxidant activity. J Neurochem 107:230–240. https://doi.org/10.1111/J.1471-4159.2008.05614.X
Bimonte S, Cascella M, Schiavone V et al (2017) The roles of epigallocatechin-3-gallate in the treatment of neuropathic pain: an update on preclinical in vivo studies and future perspectives. Drug Des Devel Ther 11:2737–2742. https://doi.org/10.2147/DDDT.S142475
Lilja S, Oldenburg J, Pointner A et al (2020) Epigallocatechin gallate effectively affects senescence and anti-SASP via SIRT3 in 3T3-L1 preadipocytes in comparison with other bioactive substances. Oxid Med Cell Longev 2020:4793125. https://doi.org/10.1155/2020/4793125
Zamami Y, Niimura T, Kawashiri T et al (2022) Identification of prophylactic drugs for oxaliplatin-induced peripheral neuropathy using big data. Biomed Pharmacother 148:112744. https://doi.org/10.1016/J.BIOPHA.2022.112744
Liu S, Uppal H, Demaria M et al (2015) Simvastatin suppresses breast cancer cell proliferation induced by senescent cells. Sci Rep 5:1–11. https://doi.org/10.1038/srep17895
Funding
Dr. Tareq Saleh is supported for this work by the Deanship of Scientific Research, The Hashemite University (grant no.743/51/2022).
Author information
Authors and Affiliations
Contributions
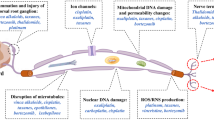
MA wrote the section on the mechanisms of CIPN. AE wrote the section on the clinical challenges associated with the treatment of CIPN. SB wrote the section on TIS. MH contributed to the writing and editing of all sections. TS wrote the section on the proposed model, designed the figure, and wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alsalem, M., Ellaithy, A., Bloukh, S. et al. Targeting therapy-induced senescence as a novel strategy to combat chemotherapy-induced peripheral neuropathy. Support Care Cancer 32, 85 (2024). https://doi.org/10.1007/s00520-023-08287-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08287-0