Abstract
Purpose
Existing fear of cancer recurrence (FCR) screening measures is being shortened to facilitate clinical use. This study aimed to evaluate the validity and screening capacity of a single-item FCR screening measure (FCR-1r) in long-term colorectal cancer (CRC) survivors with no recurrence and assess whether it performs as well in older as in younger survivors.
Methods
All Danish CRC survivors above 18, diagnosed and treated with curative intent between 2014 and 2018, were located through a national patient registry. A questionnaire including the FCR-1r, which measures FCR on a 0–10 visual analog scale, alongside the validated Fear of Cancer Recurrence Inventory Short Form (FCRI-SF) as a reference standard was distributed between November 2021 and May 2023. Screening capacity and cut-offs were evaluated with a receiver-operating characteristic analysis (ROC) in older (≥ 65 years) compared to younger (< 65 years) CRC survivors. Hypotheses regarding associations with other psychological variables were tested as indicators of convergent and divergent validity.
Results
Of the CRC survivors, 2,128/4,483 (47.5%) responded; 1,654 (36.9%) questionnaires were eligible for analyses (median age 76 (range 38–98), 47% female). Of the responders, 85.2% were aged ≥ 65. Ninety-two participants (5.6%) reported FCRI-SF scores ≥ 22 indicating clinically significant FCR. A FCR-1r cut-off ≥ 5/10 had 93.5% sensitivity and 80.4% specificity for detecting clinically significant FCR (AUC = 0.93, 95% CI 0.91–0.94) in the overall sample. The discrimination ability was significantly better in older (AUC = 0.93, 95% CI 0.91–0.95) compared to younger (0.87, 95% (0.82–0.92), p = 0.04) CRC survivors. The FCR-1r demonstrated concurrent validity against the FCRI-SF (r = 0.71, p < 0.0001) and convergent validity against the short-versions of the Symptom Checklist-90-R subscales for anxiety (r = 0.38, p < 0.0001), depression (r = 0.27, p < 0.0001), and emotional distress (r = 0.37, p < 0.0001). The FCR-1r correlated weakly with employment status (r = − 0.09, p < 0.0001) and not with marital status (r = 0.01, p = 0.66) indicating divergent validity.
Conclusions
The FCR-1r is a valid tool for FCR screening in CRC survivors with excellent ability to discriminate between clinical and non-clinical FCR, particularly in older CRC survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As more people are living longer with and after cancer, there is a growing proportion of older survivors, raising the importance of conducting research with these populations. While many long-term cancer survivors do not face the acute stressors of treatment and follow-up, they continuously deal with the uncertainties of survivorship, including potential recurrence. Fear of cancer recurrence (FCR), defined as “fear, worry or concern that cancer will come back or progress” [1] is a top health-related concern for almost all cancer survivors [2], and help managing FCR is one of the most frequently reported unmet supportive care needs [3,4,5].
Identifying survivors with clinically significant levels of FCR is important, as it can greatly affect quality of life and may persist for many years without treatment [6, 7]. On average, older long-term cancer survivors report less FCR compared to younger survivors [8], independent of cancer type [7, 9, 10], but high levels of FCR are reported in a small but significant group of older (mean age 77.6 years), long-term (average time since diagnosis 9.5 years) cancer survivors (15.9% according to a Cancer Worry Scale cut-off ≥ 14) [7].
Psychosocial screening of older cancer survivors for multiple psychosocial issues with numerous long questionnaires is problematic, as age and anticancer treatment may together and separately affect cognitive function [11]. Besides this, screening requires administration, scoring, and interpretation by a healthcare provider, all of which may be complicated by lengthy questionnaires.
Efforts have been made to create short, simple, and valid screening items. The single-item FCR measure (FCR-1) [12] with the verbal formulation “On a scale from 0 to 100, what is your subjective level of fear of cancer recurrence at this time?” was developed for use in group sessions aimed at reducing FCR. The FCR-1 has been psychometrically tested [12] and then revised and validated in written form, FCR-1r [13].
In both studies, validation was performed in relatively small samples (FCR-1; N = 69 and FCR-1r; n = 107). The psychometric properties of the FCR-1 were evaluated only in women with breast and gynecological cancer with a mean age of 55, and the FCR-1r was evaluated in a group of patients with mixed cancers where 15% had experienced a cancer recurrence. There is a need to further validate the FCR-1r in additional populations with different types of cancer and at various stages of survivorship to extend generalizability.
The primary objective of this cross-sectional study was to examine the psychometric properties of the FCR-1r, by examining its concurrent, convergent, and divergent validity in a sample of long-term colorectal cancer (CRC) survivors and to investigate the screening performance of the FCR-1r in older versus younger CRC survivors. Results can be used to support the integration of a short and simple FCR screening item into routine psychosocial screening for CRC survivors.
Materials and methods
Design
This cross-sectional study followed the COSMIN Study Design checklist for Patient-reported outcome measurement instruments [14]. The data presented is part of a national study of the prevalence of psychosocial late effects in long-term (defined as ≥ 3 years since cancer diagnosis with no recurrence and no residual disease) CRC survivors in Denmark, which subsequently will be used for recruitment to a randomized controlled trial (RCT) of a therapist-guided online FCR intervention (see published protocol) [15].
Sample and recruitment procedures
All CRC survivors in Denmark are included considering the following: (a) above 18 years; (b) diagnosed with colorectal cancer between 2014 and 2018; and (c) treated with curative intent were invited to participate in the population-based study of psychosocial late effects after CRC from which the sub-sample for this analysis was derived. CRC survivors were located through the Danish Clinical Quality Program–National Clinical Registries (RKKP) which also provided additional data (see later). For this study, data gathered between November 2021 and May 2023 was used.
The survey from “Vejle Hospital” was distributed electronically to a personal secure electronic mailbox (e-Boks) when possible, or as a paper-and-pencil survey with a cover letter presenting the study aim when patients had opted out of receiving electronic mail from the authorities. The survey included an item seeking consent for anonymous responses to be used for research. It was not possible to follow-up non-respondents. Electronic surveys were sent out in a quantity comparable to paper-and-pencil surveys (a sub-sample of 20%).
Returned questionnaires were excluded in cases with a missing social security number (for identification) or if the terms “dementia,” “blindness,” “too ill,” “cancer recurrence,” or “dead” were noted on the questionnaire or communicated to the primary investigator by telephone. In certain instances, survivors indicated “no memory of cancer,” “do not want to participate,” or “do not give consent for answers being used for research purposes,” leading to their exclusion. Participants who responded “0” to every FCRI-SF item, seemingly missing the reversed wording of item 9 “I believe that I am cured and that the cancer will not come back” were excluded, as this was interpreted as inattention or lack of motivation [16]. Paper-and-pencil questionnaire responses were manually entered into a REDCap database, with 10% double data entry for quality assurance. Responses received through e-Boks were directly uploaded into REDCap.
Data collection
The survey included clinical and demographic questions, and a battery of validated questionnaires, two of which (the Fear of Cancer Recurrence Inventory Short Form (FCRI-SF) [17] and the Symptom Checklist-90-R (SCL) [18]) were validated in Danish. The other two (the FCR-1r and the Global Quality of Life (QoL)) were translated and field-tested during this study. The translation was a collaborative effort between a trilingual research administrator and the primary investigator. It was further validated through field testing, involving an age-matched group of three ordinary people and three cancer survivors. The translation remained unchanged after the field-testing process.
The FCRI-SF [19] is the most widely validated and used FCR screening tool in a research context, but its 9-item length makes it impractical for use in routine survivorship care. Item response categories are on a 5-point Likert-like scale ranging from (0) not at all/never to (4) a great deal/all the time. Scale scores range from 0 to 36 with higher scores indicating greater FCR severity. The criterion validity of the FCRI-SF has been demonstrated in a population (n = 60, mean age 60) of short-term (mean 1.9 years) mixed cancer survivors with a face-to-face FCR interview as the gold standard [20]. Originally, two cut-off scores were validated (13/36 to indicate any level of FCR and 16/36 to indicate potential severe FCR). In this study, the more recent FCRI-SF cut-off of 22/36 was used due to its higher sensitivity (90%) and specificity (83.3%) for identifying clinical cases of FCR according to clinical interviews [21].
The FCR-1r [13] is the adaptation of the verbally administered FCR-1 [12] to a written form compatible with the Edmonton Symptom Assessment System (ESAS) [22], developed for use in routine clinical care. Respondents are asked to “Please circle the number that best describes how you feel NOW on a visual analog scale from 0 = No FCR to 10 = Worst possible FCR (FCR = fear that your cancer will come back or get worse).” In the study by Smith et al., the FCR-1r was strongly correlated to FCRI-SF (r = 0.83, p < 0.0001). As only survivors with no history of recurrence were included in the present study, “or get worse” was deleted.
The short version of the Symptom Checklist-90-R (SCL) [23] was used along with the anxiety subscale (SCL-anx) [24], depression subscale (SCL-dep), and general distress subscale (SCL-distress) [18] to measure symptoms of anxiety, depression, and emotional distress, respectively, during the previous four weeks on a 5-point Likert like scale rating from (0) not at all to (4) extremely. For each subscale, a sum score is calculated with higher scores indicating more severe symptoms. The SCL-anx, SCL-dep, and SCL-distress subscales use 4, 6, and 8 items respectively, resulting in sum scores ranging from 0 to 16, 24, and 32 respectively. The SCL has undergone psychometric testing in a primary care cohort of 701 patients as a part of the Common Mental Disorder Questionnaire (CMDQ) [25, 26]. The diagnostic performance for scores above/below cut-offs to correctly classify those with ICD-10 diagnoses according to psychiatric research interview (SCAN) was excellent. The area under the curve (AUC) for SCL-dep was 0.88 (95% CI 0.84–0.91) and for SCL-anx 0.87 (95% CI 0.82–0.92). The SCL distress also performed well, with an AUC of 0.78 (95% CI 0.71–0.85) [26].
Global QoL was rated using a single visual analog scale (VAS) inspired by the EQ-5D-5L [27]. The word “health” was changed into “quality of life,” and participants were asked to self-rate their overall quality of life TODAY on a VAS from 0 to 100 with 0 being “The worst quality of life you can imagine” and 100 being “The best quality of life you can imagine.” A comparable single-item QoL VAS showed good validity in a population of 83 patients with esophageal adenocarcinoma compared to the Medical Outcomes Study Short Form-20 and the Rotterdam Symptom Check-List [28].
Clinical and demographic questions referred to time since follow-up appointment and time to the next follow-up if not finished, chemotherapy received (yes/no), radiotherapy received (yes/no), marital status, employment status, education (any or none) citizenship (Danish or other), and children (yes/no) were also included in the survey.
Registry data
Information on participant characteristics (age and sex), clinical characteristics (cancer type (colon or rectum)), and diagnosis date and tumor stage (metastatic disease (yes/no)) were retrieved directly from the Danish Clinical Quality Program – National Clinical Registries (RKKP) along with WHO performance status at time of operation.
Statistical analysis
Analyses were performed using STATA17 (StataCorp, College Station, TX, USA). Descriptive statistics were used to summarize demographics and clinical characteristics. According to our informal criterion, an optimal rate of missing responses is less than 3% per item. If more than 15% were missing, this was considered not acceptable. In-between rates of missing data were imputed [29] with both mean imputation (imputation of participant’s own mean value of remaining answers) and multiple imputation (using mvn-algorithm and gender as auxiliary variable to impute 20 iterations of datasets) to explore differences between imputation approaches and complete case analyses. Missingness was assumed to be missing at random (MAR) [30], based on explorative analysis on a comparable population of CRC survivors (unpublished data).
Concurrent validity
For concurrent validity, associations between FCR-1r and FCRI-SF scores were assessed using Spearman’s Rho.
Construct validity was examined by testing a priori hypotheses on direction of associations between scores on the FCR-1r and relevant psychological measures. Spearman’s Rho was used as the distributions of the variables were not normal. Associated confidence intervals were calculated by bootstrapping. Difference in gender was analyzed by the Wilcoxon rank-sum test. Correlations were considered strong if > 0.70, moderate if 0.30–0.69 and low if < 0.30 [31].
Built upon rational and theoretical choices as well as previous research [2, 6, 8, 10, 32, 33], we hypothesized that the FCR-1r—similar to the FCRI-SF—would show the following:
Convergent validity
-
1.
A significant positive correlation between SCL-anx, SCL-dep, and SCL-distress.
-
2.
A weaker correlation with the SCL-dep than with the SCL-anx.
-
3.
A positive correlation between younger age and female gender, both of which are associated with greater FCR.
-
4.
A negative correlation with Global QoL.
Divergent validity
-
5.
Correlations of − 0.10 to 0.10 with marital status and employment status, i.e., no or only very small association between FCR and these demographic factors, as generally noted in the literature [34]
Screening capacity
For the two age groups and for the overall sample, receiver operating characteristic (ROC) curves evaluated the ability of the FCR-1r to discriminate between:
-
Any level of FCR (FCRI-SF ≥ 13) and no FCR
-
Potential severe FCR (FCRI-SF ≥ 16) and no potential severe FCR
-
Clinical (FCRI-SF ≥ 22) and non-clinical levels of FCR
Discrimination ability of the FCR-1r for all three cut-offs will be presented (partly in supplementary material) as applicability is dependent on the screening context.
A suggestion of an optimal cut-off score on the FCR-1r was determined based on evaluation of sensitivity, specificity, and negative (NPV) and positive predictive value (PPV), which were calculated for FCR-1r scores 0–10.
Results
Of the 4,483 CRC survivors, 2,128 (47.5%) returned the survey. 474 responses were excluded during data verification (Fig. 1). Among these were 157 cases with a response pattern of “0” to every FCRI-SF item. 1,654 participants had complete data on both FCR-1r and FCRI-SF after imputation, and were included in the analyses (median age 76 years (range 38–98), 47% females). (See Table 1 for socio-demographic and clinical characteristics for the two age groups (< 65 years and ≥ 65 years) and for the overall sample.) Mean FCR-1r score was 2.6/10 and mean FCRI-SF score was 10.1/36. Non-responders and those who were excluded were most prominent in paper surveys, but CRC survivors willing and eligible to respond were no different from CRC survivors not willing to respond regarding clinical and demographic characteristics (see Table 1a (paper population) and Table 1b (e-Boks population) in supplementary material). No unexpected differences were seen between paper and e-Boks survey respondents. E-boks responders tended to be younger and reported higher FCR levels. E-Boks responders were more likely to have rectal cancer, and a higher percentage were married (as opposed to widowed) and were still employed (see Table 2 in supplementary material).
Imputation
Only FCRI-SF data were imputed. In 163 (27%) paper questionnaires, missing values were observed in up to 4/9 FCRI-SF items (see Table 3 in supplementary material). No difference was found between results using different imputation styles (see Table 4 in supplementary material), and mean imputation was used for further statistics. After imputation, 34 cases (5.7%) reported FCRI-SF score above the cut-off of ≥ 22, but three of these cases had missing data on FCR-1r leaving 31 cases. Scores on SCL subscales were calculated only in complete cases. A unimodal distribution was observed for both FCR-1r and FCRI-SF scores with 40% and 9% reporting 0.
Concurrent validity
A strong positive correlation between FCR-1r and FCRI-SF score was observed (r = 0.71, p < 0.0001). The strongest correlation (r = 0.72, p < 0.0001) was found between FCR-1r and FCRI-SF item 1 (I am worried or anxious about the possibility of cancer recurrence), and the weakest correlation (r = 0.18, p < 0.0001) with FCRI-SF item 5 (I believe that I am cured and that the cancer will not come back). Correlations of FCRI-SF items 2–4 and 6–9 were all between 0.45 and 0.68 (see Table 5 in supplementary material).
Hypothesis testing
Convergent validity
Moderate correlations were observed between the FCR-1r and SCL-anx, SCL-dep, and SCL-distress (see Table 2). The correlation with SCL-dep was weaker than with SCL-anx. Small but significant correlations were observed between FCR-1r scores and lower age and QoL. The magnitude of correlations was similar for the FCRI-SF. Females reported higher FCR-1r and FCRI-SF scores than males (see Table 6 in supplementary material).
Divergent validity
A very small association between FCR and demographic factors was observed (see Table 2).
ROC analyses
Using the FCRI-SF cut-off of 22 or greater, there were 92 positive cases of likely clinically significant FCR in the total sample. These cases were distributed with 26 (10.6%) in the “young” group, and 66 (4.7%) in the “old” group.
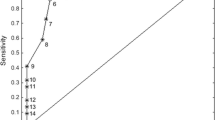
The AUC for the two age groups separately and for the total sample are presented in Fig. 2 The AUC for the total sample was 0.92 (95% CI 0.91–0.94, p < 0.0000) for the FCR-1r indicating excellent classification ability.
The AUCs were significantly different between the two age groups (< 65 years = 0.87 CI (0.82–0.92) versus ≥ 65 years = 0.93 CI (0.91–0.95), p = 0.04), indicating a better screening performance of the FCR-1r in the older group.
A cut-off score of ≥ 5/10 on the FCR-1r was deemed best suited to screen for likely clinically significant FCR with a sensitivity of 93.5% and a specificity of 80.3% (see Table 3). At this cut-off, the NPV was 99.5% and the PPV was 21.9%.
Using the FCRI-SF cut-off of 16 or greater [20] indicating potential severe FCR, there were 349 positive cases. The AUC was 0.85 (95% CI 0.83–0.87, p < 0.0000) for the FCR-1r indicating good classification ability. Using the FCRI-SF cut-off of 13 or greater [20] as a rapid screening measure, there were 605 positive cases. The AUC was 0.85 (95% CI 0.83–0.86, p < 0.0000) for the FCR-1r indicating good classification ability. For ROC curves and accuracy measures of these cut-offs see Figs. 1 and 2 in supplementary material.
Discussion
Valid psychosocial screening instruments with clinically relevant cut-offs are crucial to personalizing survivorship care. This study aimed to assess the validity of the FCR-1r and evaluate its screening performance in older versus younger CRC survivors with no recurrence of cancer. The FCR-1r demonstrated satisfactory concurrent, convergent, and divergent validity compared to the FCRI-SF and measures of constructs related to FCR. A FCR-1r cut-off score ≥ 5 provided an optimal balance of sensitivity, specificity, NPV, and PPV for identifying likely cases of clinically significant FCR (FCRI-SF ≥ 22) in practice. The validity of the FCR-1r was comparable to the original FCR-1 [12], and the classification ability in this population seems adequate.
While this study provides important evidence regarding the validity and screening performance of the FCR-1r in a population of cancer survivors with lower rates of clinical FCR compared to previous findings in CRC survivors [17, 35], further work is needed to confirm the unusually low prevalence of clinical FCR in older populations with different health care systems and to confirm the utility of the FCR-1r as a screening tool for FCR across different survivorship stages, especially in patients with recurrent disease.
Short screening questionnaires can advantageously be followed by longer and more detailed measurements like the FCRI-SF in a two-step screening process to confirm FCR severity, as recommended for depression [36]. In the current context, the high specificity is particularly reassuring, as only 20% of patients (i.e., those reporting false positives) would go through this step unnecessarily. The NPV is extremely high regardless of cut-off, due to the low number of cases in this cohort [37], but the low PPV underpins the need for a two-step approach.
Other globally used psycho-oncology screening measures like the Distress Thermometer (DT) and the ESAS assess fears and anxiety, but not specifically FCR and FCR might occur as an isolated focus of worry not captured by these. For example, high overall distress and a positive fear item on the problem list on the DT [38] showed low NPV and sensitivity to detect high FCR (AUC around 0.70 depending on cut-off on the Cancer Worry Scale (CWS-6)) [39], indicating that a high score could be due to high FCR, but could also reflect other causes of distress, and a low proportion of patients with high FCR would be identified using the DT. No subsequently detailed measurements can detect cases if they do not proceed to this next step, and the DT was deemed unsuitable for FCR screening in routine practice.
In contrast, the ESAS-r [22] anxiety item showed surprisingly good screening performance (AUC = 0.87) in detecting likely cases of clinical FCR in the FCR-1r validation study by Smith et al. [13], comparable to the FCR-1r (AUC = 0.89) and the FCR-1r in our study (AUC = 0.92). Not all patients might recognize FCR as fear, as this generic wording could also be interpreted more broadly as related to the impact of cancer on health, family, or work rather than recurrence in particular, arguably anxiety and FCR seems closer related.
If FCR-1r was expected to be a stand-alone question, it could be just as easy to ask the survivor about FCR orally at the medical encounter, but this rarely happens [40, 41]. Therefore, routine screening is recommended. FCR is only one of many other late effects present among long-term cancer survivors, such as general distress, cognitive impairment, sexual dysfunction, financial toxicity, and fatigue [42], and a larger battery of screening measures should be included in the psychosocial screening. The FCR-1r is valid, time-efficient, and simple to interpret. It can be easily integrated into existing screening procedures in routine survivorship care, and it gives a clear indication of whether it is relevant to spend time at the consultation discussing FCR and possible treatment options.
Limitations
Missing data was an issue for the paper-and-pencil questionnaires and we also excluded more than 150 responses, with a response pattern of “0.” The fairly large number of responses excluded due to consistent responses despite the reversed item is not ideal. The strategy of imputation was to test two different methods and decide which one to proceed with, based on the impact on the datasets and on the analyses, and compared to complete case analyses. Multiple imputation might be a more powerful and elegant strategy, but besides being more comprehensive, the subsequent STATA prefix “mi estimate” limits the variety of analyses supported by STATA. Mean imputation is easily applicable in clinical and research perspectives and our results confirm the validity of this more accessible strategy, as no difference was observed between imputation styles on the interrelationship between FCRI-SF and FCR-1r. Mean imputation of the participant’s own mean can be used in future studies with missing FCRI-SF data.
As all CRC survivors in Denmark diagnosed between 2014 and 2018 were invited to participate in this study without indicating any sign of interest, the response rate was expected to be low. We do not have registry data on recurrence, but estimate, that about 25% of our population was not eligible for participation due to cancer recurrence. If these were excluded a priori we would reach a response rate of around 60%, consistent with comparable research in this field. Future studies could overcome this by seeking insight into medical records and only approach CRC survivors with no recurrence and no residual disease, or by including people living with or after recurrence of cancer.
Denmark is a highly digitalized country, and all strictly personal communication from the authorities is electronic unless you apply to receive messages by post. Digital literacy is closely, but not completely, related to age, and to comprehensively investigate the FCR level in all CRC survivors, we needed to distribute both paper and electronic surveys. Differences in respondents according to survey type were expected and corresponded to age and age-related characteristics like FCR level, employment status, marital status, education, and the clinical characteristics of cancer type and chemotherapy. Perhaps more importantly there were no differences in registry data between respondents and non-respondents, suggesting that our sample was representative. We have no reason to believe the severity of FCR is related to digital literacy, but this could be further investigated in future studies, especially as there are a growing number of online FCR interventions (e.g., iConquerFear).
Choosing another self-report measure as a gold standard is troublesome, as this measure too has limited accuracy for correctly classifying patients with clinical FCR. Investigating the capacity of the FCR-1r alone compared to a two-step model incorporating the FCR-1r against a gold-standard clinical interview would shed some light on which strategy is most suitable when considering burden on both health professionals and patients.
We focused on validating and testing the screening performance of a short FCR screening tool with high sensitivity and NPV for identifying clinically significant FCR in long-term CRC survivors with minimal patient burden, considering the relatively low prevalence of clinical FCR in this population. However, we note that the FCR-1r also demonstrated good classification ability in identifying less severe levels of FCR, which may be relevant to contexts where patient burden and resources available to provide intervention for lower FCR levels are less of a concern.
Conclusion
Although the prevalence of patients reporting likely clinical FCR (FCRI-SF score ≥ 22) was low among long-term CRC survivors in our sample, particularly those who were older, the FCR-1r screening tool appears to be useful in identifying those who are likely to have clinical levels of FCR. The results suggest that using a cut-off score of ≥ 5 on the FCR-1r tool is appropriate for selecting survivors who may require further assessment and treatment.
Data availability
The dataset generated during this study is available from the corresponding author upon request.
References
Lebel S, Ozakinci G, Humphris G, Mutsaers B, Thewes B, Prins J et al (2016) From normal response to clinical problem: definition and clinical features of fear of cancer recurrence. Supp Care Cancer 24(8):3265–3268. https://doi.org/10.1007/s00520-016-3272-5
Simard S, Thewes B, Humphris G, Dixon M, Hayden C, Mireskandari S et al (2013) Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv 7(3):300–322. https://doi.org/10.1007/s11764-013-0272-z
Harrison SE, Watson EK, Ward AM, Khan NF, Turner D, Adams E et al (2011) Primary health and supportive care needs of long-term cancer survivors: a questionnaire survey. J Clin Oncol 29(15):2091–2098. https://doi.org/10.1200/jco.2010.32.5167
Armes J, Crowe M, Colbourne L, Morgan H, Murrells T, Oakley C et al (2009) Patients’ supportive care needs beyond the end of cancer treatment: a prospective, longitudinal survey. J Clin Oncol 27(36):6172–6179. https://doi.org/10.1200/jco.2009.22.5151
Kotronoulas G, Papadopoulou C, Burns-Cunningham K, Simpson M, Maguire R (2017) A systematic review of the supportive care needs of people living with and beyond cancer of the colon and/or rectum. European J Oncol Nursing 29:60–70. https://doi.org/10.1016/j.ejon.2017.05.004
Koch L, Jansen L, Brenner H, Arndt V (2013) Fear of recurrence and disease progression in long-term (≥ 5 years) cancer survivors–a systematic review of quantitative studies. Psychooncology 22(1):1–11. https://doi.org/10.1002/pon.3022
Krok-Schoen JL, Naughton MJ, Bernardo BM, Young GS, Paskett ED (2018) Fear of recurrence among older breast, ovarian, endometrial, and colorectal cancer survivors: Findings from the WHI LILAC study. Psychooncology 27(7):1810–1815. https://doi.org/10.1002/pon.4731
Crist JV, Grunfeld EA (2013) Factors reported to influence fear of recurrence in cancer patients: a systematic review. Psychooncology 22(5):978–986. https://doi.org/10.1002/pon.3114
Deimling GT, Bowman KF, Sterns S, Wagner LJ, Kahana B (2006) Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psychooncology 15(4):306–320. https://doi.org/10.1002/pon.955
Koch-Gallenkamp L, Bertram H, Eberle A, Holleczek B, Schmid-Höpfner S, Waldmann A et al (2016) Fear of recurrence in long-term cancer survivors-Do cancer type, sex, time since diagnosis, and social support matter? Health Psychol 35(12):1329–1333. https://doi.org/10.1037/hea0000374
Regier NG, Naik AD, Mulligan EA, Nasreddine ZS, Driver JA, Sada YH et al (2019) Cancer-related cognitive impairment and associated factors in a sample of older male oral-digestive cancer survivors. Psychooncology 28(7):1551–1558. https://doi.org/10.1002/pon.5131
Rudy L, Maheu C, Körner A, Lebel S, Gélinas C (2020) The FCR-1: initial validation of a single-item measure of fear of cancer recurrence. Psychooncology 29(4):788–795. https://doi.org/10.1002/pon.5350
Smith A, Gao M, Tran M, Ftanou M, Jegathees S, Wu V et al (2023) Evaluation of the validity and screening performance of a revised single-item fear of cancer recurrence screening measure (FCR-1r). Psychooncology 32(6):961–971. https://doi.org/10.1002/pon.6139
Mokkink LB, Prinsen C, Patrick DL, Alonso J, Bouter LM, De Vet H et al (2019) COSMIN Study Design checklist for patient-reported outcome measurement instruments. The Netherlands, Amsterdam, pp 1–32
Lyhne JD, Smith AB, Frostholm L, Fink P, Jensen LH (2020) Study protocol: a randomized controlled trial comparing the efficacy of therapist guided internet-delivered cognitive therapy (TG-iConquerFear) with augmented treatment as usual in reducing fear of cancer recurrence in Danish colorectal cancer survivors. BMC Cancer 20(1):223. https://doi.org/10.1186/s12885-020-06731-6
van Sonderen E, Sanderman R, Coyne JC (2013) Ineffectiveness of reverse wording of questionnaire items: let’s learn from cows in the rain. PLoS ONE 8(7):e68967. https://doi.org/10.1371/journal.pone.0068967
Hovdenak Jakobsen I, Jeppesen MM, Simard S, Thaysen HV, Laurberg S, Juul T (2018) Initial validation of the Danish version of the Fear of Cancer Recurrence Inventory (FCRI) in colorectal cancer patients. J Cancer Surviv 12(6):723–732. https://doi.org/10.1007/s11764-018-0709-5
Olsen LR, Mortensen EL, Bech P (2004) The SCL-90 and SCL-90R versions validated by item response models in a Danish community sample. Acta Psychiatr Scand 110(3):225–229. https://doi.org/10.1111/j.1600-0447.2004.00399.x
Simard S, Savard J (2009) Fear of Cancer Recurrence Inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Supp Care Cancer 17(3):241–251. https://doi.org/10.1007/s00520-008-0444-y
Simard S, Savard J (2015) Screening and comorbidity of clinical levels of fear of cancer recurrence. J Cancer Surviv 9(3):481–491. https://doi.org/10.1007/s11764-015-0424-4
Fardell JE, Jones G, Smith AB, Lebel S, Thewes B, Costa D et al (2018) Exploring the screening capacity of the Fear of Cancer Recurrence Inventory-Short Form for clinical levels of fear of cancer recurrence. Psychooncology 27(2):492–499. https://doi.org/10.1002/pon.4516
Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K (1991) The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 7(2):6–9
Derogatis LR, Cleary PA (1977) Confirmation of the dimensional structure of the SCL-90: A study in construct validation. J Clin Psychol 33(4):981–989
Christensen KS, Fink P, Toft T, Frostholm L, Ornbøl E, Olesen F (2005) A brief case-finding questionnaire for common mental disorders: the CMDQ. Fam Pract 22(4):448–457. https://doi.org/10.1093/fampra/cmi025
Christensen KS, Bech P, Fink P (2010) Measuring mental health by questionnaires in primary care-unidimensionality, responsiveness and compliance. Eur Psychiatr Rev 3(1):8–12
Christensen KS, Fink P, Toft T, Frostholm L, Ørnbøl E, Olesen F (2005) A brief case-finding questionnaire for common mental disorders: the CMDQ. Fam Pract 22(4):448–457. https://doi.org/10.1093/fampra/cmi025
Rabin R, de Charro F (2001) EQ-5D: a measure of health status from the EuroQol Group. Ann Med 33(5):337–343. https://doi.org/10.3109/07853890109002087
de Boer AG, van Lanschot JJ, Stalmeier PF, van Sandick JW, Hulscher JB, de Haes JC et al (2004) Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual Life Res 13:311–320
De Vet HC, Terwee CB, Mokkink LB, Knol DL (2011) Measurement in medicine: a practical guide: Cambridge University Press
Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG et al (2009) Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. Bmj. 338:b2393. https://doi.org/10.1136/bmj.b2393
Akoglu H (2018) User’s guide to correlation coefficients. Turkish J Emerg Med 18(3):91–93
Lim E, Humphris G (2020) The relationship between fears of cancer recurrence and patient age: a systematic review and meta-analysis. Cancer Reports (Hoboken, NJ) 3(3):e1235. https://doi.org/10.1002/cnr2.1235
Pang C, Humphris G (2021) The relationship between fears of cancer recurrence and patient gender: a systematic review and meta-analysis. Front Psychol 12:640866. https://doi.org/10.3389/fpsyg.2021.640866
Luigjes-Huizer YL, Tauber NM, Humphris G, Kasparian NA, Lam WWT, Lebel S et al (2022) What is the prevalence of fear of cancer recurrence in cancer survivors and patients? A systematic review and individual participant data meta-analysis. Psychooncology 31(6):879–892. https://doi.org/10.1002/pon.5921
Custers JAE, Gielissen MFM, Janssen SHV, de Wilt JHW, Prins JB (2016) Fear of cancer recurrence in colorectal cancer survivors. Supp Care Cancer 24(2):555–562. https://doi.org/10.1007/s00520-015-2808-4
Mitchell AJ, Meader N, Davies E, Clover K, Carter GL, Loscalzo MJ et al (2012) Meta-analysis of screening and case finding tools for depression in cancer: evidence based recommendations for clinical practice on behalf of the Depression in Cancer Care consensus group. J Affect Disord 140(2):149–160. https://doi.org/10.1016/j.jad.2011.12.043
Juul S (2006) An introduction to Stata for health researchers: STATA press
Network N (2019) NCCN Clinical Practice Guidelines in Oncology: Distress Management V. 3
Deuning-Smit E, Custers JAE, Kwakkenbos L, Hermens R, Prins JB (2022) Evaluating the capacity of the distress thermometer to detect high fear of cancer recurrence. Psychooncology. https://doi.org/10.1002/pon.6066
Liu JJ, Butow P, Beith J (2019) Systematic review of interventions by non-mental health specialists for managing fear of cancer recurrence in adult cancer survivors. Supp Care Cancer 27(11):4055–4067. https://doi.org/10.1007/s00520-019-04979-8
Smith AB (2021) Integrating fear of cancer recurrence screening into routine care: opportunities and challenges. Psychooncology 30(1):134–137. https://doi.org/10.1002/pon.5558
van Leeuwen M, Husson O, Alberti P, Arraras JI, Chinot OL, Costantini A et al (2018) Understanding the quality of life (QOL) issues in survivors of cancer: towards the development of an EORTC QOL cancer survivorship questionnaire. Health Qual Life Outcomes 16(1):114. https://doi.org/10.1186/s12955-018-0920-0
Acknowledgements
Thanks to Harald Hammershøi and OPEN, Odense Patient data Explorative Network, Odense University Hospital, Odense, Denmark: www.sdu.dk/ki/open, for assistance with REDCap and to all the colorectal cancer survivors who participated in the study.
Funding
Open access funding provided by Odense University Hospital JDL was supported by the Danish Cancer Society, Grant# R280 A16561. ABS was funded by the New South Wales (NSW) Government through a Cancer Institute NSW Career Development Fellowship (#2021/DCF1138).
Author information
Authors and Affiliations
Contributions
Johanne Dam Lyhne, Allan “Ben” Smith, Lisbeth Frostholm, Lars Henrik Jensen, and Per Fink contributed to the study conception and design. Data collection was performed by Johanne Dam Lyhne. Statistical analysis was performed by Johanne Dam Lyhne and assisted by a statistician, Signe Timm. The first draft of the manuscript was written by Johanne Dam Lyhne in close cooperation with Allan “Ben” Smith. The imputation strategy was decided by Johanne Dam Lyhne, Sébastien Simard, and Signe Timm. The validation strategy was decided by Johanne Dam Lyhne, Allan “Ben” Smith, and Per Fink. All authors have revised previous versions of the manuscript critically and approved the final version for publication.
Corresponding author
Ethics declarations
Ethics approval
This study was performed according to the principles of the Declaration of Helsinki. The project was approved on the 20th of August 2019 by The Regional Committees on Health Research Ethics for Southern Denmark (Project-ID: S-20190061).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publication
The authors affirm that participants provided informed consent for the publication of anonymized data.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lyhne, J.D., Smith, A.“., Timm, S. et al. Validity and screening capacity of the FCR-1r for fear of cancer recurrence in long-term colorectal cancer survivors. Support Care Cancer 31, 690 (2023). https://doi.org/10.1007/s00520-023-08159-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08159-7