Abstract
Purpose
Clinical practice guidelines recommend the use of all approved granulocyte colony-stimulating factors (G-CSFs), including filgrastim and pegfilgrastim, as primary febrile neutropenia (FN) prophylaxis in patients receiving high- or intermediate-risk regimens (in those with additional patient risk factors). Previous studies have examined G-CSF cost-effectiveness by cancer type in patients with a high baseline risk of FN.
This study evaluated patients with breast cancer (BC), non-small cell lung cancer (NSCLC), or non-Hodgkin’s lymphoma (NHL) receiving therapy who were at intermediate risk for FN and compared primary prophylaxis (PP) and secondary prophylaxis (SP) using biosimilar filgrastim or biosimilar pegfilgrastim in Austria, France, and Germany.
Methods
A Markov cycle tree-based model was constructed to evaluate PP versus SP in patients with BC, NSCLC, or NHL receiving therapy over a lifetime horizon. Cost-effectiveness was evaluated over a range of willingness-to-pay (WTP) thresholds for incremental cost per quality-adjusted life year (QALY) gained. Sensitivity analyses evaluated uncertainty.
Results
Results demonstrated that using biosimilar filgrastim as PP compared to SP resulted in incremental cost-effectiveness ratios (ICERs) well below the most commonly accepted WTP threshold of €30,000. Across all three countries, PP in NSCLC had the lowest cost per QALY, and in France, PP was both cheaper and more effective than SP. Similar results were found using biosimilar pegfilgrastim, with ICERs generally higher than those for filgrastim.
Conclusions
Biosimilar filgrastim and pegfilgrastim as primary prophylaxis are cost-effective approaches to avoid FN events in patients with BC, NSCLC, or NHL at intermediate risk for FN in Austria, France, and Germany.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Febrile neutropenia (FN) is associated with significant morbidity in patients receiving myelosuppressive chemotherapy [1,2,3,4]. Mortality is also impacted through the direct health risks of FN and through the indirect effect of FN on delays and dose reductions of the patient’s active cancer treatment [5]. In addition, the costs and effects on hospital cancer services are significant. In the USA, FN is responsible for 5.2% of all cancer-related hospitalisations [6]. The risk and severity of FN depend on the chemotherapy regimen, dose intensity, and patient-specific risk factors [7]. Better prevention of FN could potentially benefit patients and healthcare systems at multiple levels.
Access to effective prevention of FN by granulocyte colony-stimulating factors (G-CSFs) is often limited by the high cost of these biologic medicines, resulting in restricted reimbursement by multiple health technology assessments (HTA) to only a subset of all the patients who could potentially benefit [8]. However, the loss of patent protection has enabled biosimilar versions of G-CSFs to be approved, creating competition to check prices. Simultaneously, real-world data on the performance of different formulations of G-CSFs have become available [9]. Together, this signals a need to reappraise the cost-effectiveness of traditional reimbursement decisions in this treatment space [10].
Clinical guidelines published by the European Society for Medical Oncology recommend the use of G-CSFs, including short-acting (filgrastim) and long-acting (pegfilgrastim) and approved biosimilars, as primary prophylaxis (PP) for FN in patients receiving high-risk regimens (> 20% risk of FN) or intermediate-risk regimens (> 10% risk) in the presence of additional patient risk factors [11]. Previously published studies have examined the cost-effectiveness of G-CSFs by cancer type; however, these have focused on patients with a high risk of FN [12,13,14].
In the USA, a study [15] evaluating the cost-effectiveness of G-CSFs in FN in patients receiving intermediate-risk first-line, curative-intent chemotherapy for breast cancer (BC), non-small cell lung cancer (NSCLC), or non-Hodgkin’s lymphoma (NHL) concluded that biosimilar filgrastim as PP compared to secondary prophylaxis (SP) provided an additional 0.102–0.144 life years (LY) and 0.065–0.130 quality-adjusted life years (QALY) at incremental costs ranging from $650–$2463 USD. To the best of our knowledge, no similar work has been published in Western Europe.
Based on this paucity of European data, the objective of this study was to build on the prior research in the USA to evaluate a similar European population of patients and compare PP and SP for FN using either the G-CSF biosimilar or respective reference product in patients at intermediate risk of FN. To represent the diversity of European healthcare, three different systems were included: Austria, with a two-tier system of centrally funded care and additional supplementary health insurance; France, with a centrally funded universal healthcare system; and Germany, with approximately 1100 public or private sickness funds with co-payments to prevent overutilisation and control costs [16].
Methods
Model structure
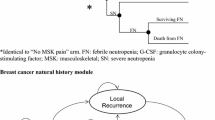
Building on the previously published cost-effectiveness model [15], a Markov cycle tree-based model was constructed in Microsoft® Excel® to evaluate the cost-effectiveness of PP versus SP with biosimilar filgrastim and biosimilar pegfilgrastim from the perspective of public health in Austria, France, and Germany [12, 13, 15].
The model structure is shown in Fig. 1 and was adapted to the specific number of cycles for each cancer type.
The first cycle was structured as a decision tree to pursue either a PP or SP strategy. The per-cycle risk of FN was estimated by distributing the baseline risk of FN over the full course of chemotherapy, with greater risk of FN in cycle 1 than subsequent cycles [17]. If an FN event occurred, all patients were treated in an inpatient setting and were at risk of dying or surviving to cycle 2 of chemotherapy. All cycle 1 deaths were assumed to be FN-related and occurred at the end of the cycle.
Chemotherapy cycles 2–6 consisted of a Markov cycle tree. In each 3-week cycle, patients entered with or without a history of FN. As in cycle 1, patients were at risk for FN, and if an FN event occurred, patients were treated as an inpatient and could survive to the next cycle or die from FN. All deaths in cycles 2–6 were assumed to be FN-related and to occur at the end of the cycle. Surviving patients repeated the Markov cycle until completing cycle 6. If patients had a history of FN from a prior cycle, the patient’s relative risk (RR) of FN would increase in subsequent cycles.
The post-chemotherapy phase consisted of 1-year Markov cycles. Initially, patients diverged into 2 groups based on cancer-specific risk considering their relative dose intensity (RDI) of chemotherapy and history of FN. Patients with a lower RDI were at higher annual risk of cancer-related death. Surviving patients re-entered the Markov cycle annually. After 20 years post-chemotherapy, mortality reverted to standard age- and sex-related rates as it was assumed patients were cured [18, 19]. The model considered a lifetime horizon.
To evaluate PP compared to SP with biosimilar filgrastim and biosimilar pegfilgrastim, incremental cost-effectiveness ratios (ICERs) were calculated for costs per FN event avoided, per LY gained, and per QALY gained. Cost-effectiveness was assessed at the commonly cited willingness-to-pay (WTP) threshold of €30,000 using SP as the reference comparator [20].
Model parameters
Clinical, utility, and mortality inputs are presented in Table 1. In each analysis, the age at which patients entered the cohort varied according to their cancer type [4, 15]. Similarly, the baseline FN risks for each cancer type were selected to reflect the real world and focus on intermediate risk [4, 15].
Disease-specific chemotherapy regimens associated with intermediate risk of FN were considered including docetaxel and cyclophosphamide (TC) in BC; rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) in NHL; and carboplatin and paclitaxel in NSCLC. Base-case inputs for the effectiveness of filgrastim and pegfilgrastim versus no G-CSF in reducing the risk of FN per cycle were based on a meta-analysis evaluating trials of G-CSF in solid tumours by cancer type [14]. The RR of FN for filgrastim was 0.42; for pegfilgrastim, 0.25; these were assumed to be the same for BC, NSCLC, and NHL [15, 21].
As the risk for FN over the entire course of chemotherapy is greatest in the first cycle, baseline cycle-specific FN risk was calculated as the risk in patients without a history of FN and without G-CSF prophylaxis. This baseline risk was decreased in patients who received G-CSF prophylaxis [12, 15]. History of FN increased the likelihood of subsequent FN events [15].
Health benefits in QALYs were calculated by adjusting LYs with utility values for, during, and after chemotherapy. Utility values were sourced from previously published cost-effectiveness analyses [12, 13, 15]. A hospitalisation for FN was expected to have a significant impact on quality of life while receiving chemotherapy. After all cycles of chemotherapy are complete, quality of life for the first year was expected to improve, with greater improvement after 1-year post-chemotherapy [12, 13, 15, 22,23,24]. Post-chemotherapy utility was assumed to remain constant until death.
Although long-term cancer survival rates vary across the three cancer types, long-term relapse rates are not influenced by G-CSF usage. Therefore, mortality rates over 1 year were based on 5-year survival rates adjusted to a 1-year probability of death for all cancer types. The model assumed the cancer-specific mortality rate was additive to the age- and sex-specific standard mortality rates for each country and assumed to only apply during the first 20 years following chemotherapy, after which the patient reverted to the standard country-specific age- and sex-specific mortality rates, as previously described [12].
A summary of cost inputs in the model is shown in Online Resource 1. Costs included in the model were treatment with biosimilar filgrastim and biosimilar pegfilgrastim and inpatient FN management. The cost of G-CSF administration was assumed to be included in the cost of inpatient admission. Chemotherapy costs were excluded from the analysis as they were assumed to be equivalent between patients receiving PP and SP. Similarly, the difference in chemotherapy costs for patients receiving low versus high RDI was assumed to be negligible. Post-chemotherapy costs were assumed to be unaffected by prophylaxis strategy and were therefore excluded. Drug costs were based on published list price information per country for a representative biosimilar filgrastim and representative biosimilar pegfilgrastim.
Sensitivity analysis
Alternative parameter values were tested via a one-way sensitivity analysis (OWSA) to evaluate the impact of each parameter on the models’ outcomes. In addition, a probabilistic sensitivity analysis (PSA) accounted for joint uncertainty among all model parameters and assessed the likelihood of cost-effectiveness of PP over a range of WTP thresholds. The PSA simulated 1000 iterations with parameter values sampled simultaneously from their individual distributions.
Results
Results are presented separately by cancer, country, and filgrastim/pegfilgrastim in Table 2 (Austria), Table 3 (France), and Table 4 (Germany) and are summarised as follows.
Austria
Base-case results for Austria show that across the three cancers, biosimilar filgrastim as PP versus SP provided an additional 0.073–0.154 LYs and 0.063–0.139 QALYs and prevented 0.100–0.118 FN events. The highest gains were for patients with NHL and the lowest gains were for patients with BC. Incremental cost ranged from €111–€644. The ICERs ranged from €972–€5840 per FN event avoided, €758–€8029 per LY gained, and €1069–€9219 per QALY gained, with NSCLC reflecting the lowest ICERs.
According to the OWSA for cost per QALY gained (Online Resource 2), for NSCLC, variation in baseline risk of FN, mean hospital length of stay (LOS) for FN, and RR of FN for filgrastim had the greatest influence on the results. For BC and NHL, variation in baseline risk of FN, mortality hazard ratio for RDI, and RR of FN for filgrastim were the most influential.
For pegfilgrastim, incremental LY gains, QALY gains, and FN events avoided were slightly higher than for filgrastim (0.090–0.196 LYs and 0.079–0.177 QALYs gained and 0.123–0.142 FN events prevented). As with filgrastim, the highest gains were for patients with NHL, and the lowest gains were for patients with BC. Incremental costs were higher than those for filgrastim, ranging from €285–€965. The ICERs ranged from €2036–€6781 per FN event avoided, €1558–€9205 per LY gained, and €2199–€10,568 per QALY gained, with NSCLC reflecting the lowest ICERs.
According to the OWSA on the cost per QALY gained, for NSCLC variation in baseline risk of FN, mean length of stay for a hospitalisation and RR of FN for pegfilgrastim had the greatest influence on the results. For BC variation in baseline risk of FN, mortality hazard ratio of RDI and discount rate had the greatest influence on the results; for NHL, the inputs with the greatest influence were baseline risk of FN, the mortality hazard ratio of RDI, and RR of FN for pegfilgrastim.
France
Base-case results for France show that across the three cancers, biosimilar filgrastim as PP versus SP provided an additional 0.073–0.145 LYs and 0.063–0.131 QALYs. As efficacy inputs are the same across all three countries, FN events prevented were the same as those for Austria. QALY and LY results differed from those in Austria due to country-specific mortality data. The highest gains were for patients with NHL and lowest were for patients with BC. Incremental costs ranged from savings of €152 for NSCLC to €758 for NHL. The ICERs for NHL and BC were €6421 and €4489 per FN event avoided, €5248 and €6211 per LY gained, and €5807 and €7131 per QALY gained, respectively. For NSCLC, PP was both cheaper and more effective than SP and therefore PP dominated SP.
According to the OWSA for cost per QALY gained (Online Resource 3), for NSCLC variation in baseline risk of FN, mean LOS when hospitalised for FN and RR of FN for filgrastim were the only inputs that resulted in PP not dominating SP. For BC variation in baseline risk of FN, mortality hazard ratio for RDI and RR of FN for filgrastim had the greatest influence on the results. For NHL, the inputs with the greatest influence were baseline risk of FN, RR of FN for filgrastim, and the patient’s weight.
For pegfilgrastim, PP provided an additional 0.089–0.184 LYs and 0.078–0.166 QALYs. The highest gains were for patients with NHL, and lowest were in patients with BC. Incremental costs ranged from €258–€990. The ICERs ranged from €1839–€9358 per FN event avoided, €1499–€11,076 per LY gained, and €2112–€12,715 per QALY gained, with NSCLC reflecting the lowest ICERs.
According to the OWSA for cost per QALY gained, for NSCLC variation in baseline risk of FN, mean LOS for a hospitalisation and RR of FN for pegfilgrastim had the greatest influence on the results. For BC and NHL variation in baseline risk of FN, the mortality hazard ratio of RDI and RR of FN for pegfilgrastim had the greatest influence on the results.
Germany
Base-case results for Germany show that across the three cancers, biosimilar filgrastim as PP versus SP provided an additional 0.078–0.145 LYs and 0.067–0.141 QALYs. The highest gains were for patients with NHL and lowest were in patients with BC. Incremental costs were higher than in both Austria and France, ranging from €1551–€1886. Due to these higher incremental costs, ICERs in Germany are higher for all three cancer types. The cost per FN event avoided ranged from €13,550–€20,909, the cost per LY gained ranged from €10,698–€24,334, and the cost per QALY gained ranged from €15,090–€27,960, with NSCLC reflecting the lowest ICERs.
Similar to Austria and France, the OWSA showed that for NSCLC variation in baseline risk of FN (Online Resource 4), RR of FN for filgrastim and cost of an FN event hospitalisation had the greatest influence on results. For BC variation in baseline risk of FN, the mortality hazard ratio for RDI and the discount rate had the greatest influence on results. For NHL, the inputs with the greatest influence were baseline risk of FN, RR of FN for filgrastim, and the patient’s weight.
For pegfilgrastim, PP provided an additional 0.095–0.199 LYs and 0.083–0.179 QALYs. The highest gains were for patients with NHL and the lowest were in patients with BC. Incremental costs ranged from €3073–€4658. The ICERs ranged from €21,932–€32,746 per FN event avoided, €17,003–€35,951 per LY gained, and €23,990–€41,305 per QALY gained, with NSCLC reflecting the lowest ICERs. As for filgrastim, ICERs were higher in Germany than in Austria and France
According to the OWSA on the cost per QALY gained, for NSCLC variation in baseline risk of FN, RR of FN for pegfilgrastim and the discount rate had the greatest influence on the results. For BC and NHL variation in baseline risk of FN, the mortality hazard ratio of RDI and RR of FN for pegfilgrastim had the greatest influence on the results.
PSA results
The PSA identified that for filgrastim, at a WTP threshold of €50,000 per QALY gained, the probability of the cost-effectiveness of PP remained high across all three cancer types for all three countries (ranging from 95%–100%). Similar results were shown for pegfilgrastim (96%–100%).
Discussion
Europe has 18 different versions of filgrastim or pegfilgrastim, either as originator or as biosimilar brand [25]. This has been sufficient to change cost-effectiveness assumptions behind European guidelines—as this study shows. American studies are in agreement, confirming that while the costs of the FN events remain high, lower drug costs support the concept that PP may be cost-effective at even modest spending thresholds [26,27,28].
Based on this analysis, expanding reimbursement to cover biosimilar filgrastim or pegfilgrastim for patients treated with intermediate-risk chemotherapy regimens would be considered cost-effective. This is an important consideration also in view of the present COVID crisis and appeals of learned societies for a more liberal use of preventive growth factors [29]. It resulted in ICERs well below the commonly used WTP threshold of €30,000. Across all three countries, NSCLC had the lowest cost per QALY, and in France, PP dominated SP such that PP was considered both cheaper and more effective. Similar results were found using pegfilgrastim, with ICERs generally higher than those for filgrastim.
This base-case assessment used current ex-manufacturer list prices, which is common practice when undertaking an HTA. In Austria, however, pricing is dependent on whether a drug is prescribed at the initial clinic visit, when treatment is covered under a disease-related group payment, or after this when the pharmacy sale list price is considered. Scenario analysis at the upper limit pricing for filgrastim (€55.54 per 300 mcg) and pegfilgrastim (€430.75 per dose) showed that PP remains cost-effective across all three cancer types for both filgrastim and pegfilgrastim. In France, tendering is common practice. As such, prices are likely to be lower than the list price used in the base case. Scenario analysis was performed for a 30% discount for biosimilar filgrastim (€40) and 15% for biosimilar pegfilgrastim (€400); as expected given a lower price, PP remains cost-effective. Importantly, past experience suggests that real-world costs will fall and cost-effectiveness will rise still further as the competitive market for medicines created by biosimilars creates further tender-based discounts [30].
The value of G-CSFs changes over time as research identifies patient groups at greater chance of clinical benefit while prices can change due to competition [8]. To remain relevant, guidelines based around cost-effectiveness estimates, such as the current US and European guidelines for G-CSF use, must also change over time. The conventional perception has been that G-CSFs are traditionally expensive drugs that should be conserved for perceived high-value patient scenarios, e.g. patients at high risk of FN, who theoretically incur higher incidence rates and subsequent cost burdens with episodes of FN. This perception is now challenged by the findings that biosimilars deliver significant cost savings related to the number of competing brands and time since biosimilar launches in both Europe and the USA [31, 32], with the focus of treatment changing to ensuring that guidelines are met [33, 34].
Furthermore, previous studies of biosimilar treatments in Europe have shown that the benefits of biosimilars are not limited to costs savings and may expand access to treatment and improve cost-effectiveness of the treatment [35]. The results of the modelling presented here have shown similar benefits in the use of biosimilar treatments.
There are some limitations to this study. First, the structure of the analyses likely represents a simplification of the complex interplay between disease, treatments, and costs. For example, our model considered the FN risk associated with the regimen but was unable to consider the FN risk of individual patients as we were limited by available data inputs. Some patients may have been treated in the outpatient setting based on individual risk factors instead of in the hospital, which would likely be less costly. However, multiple studies have suggested that, while patient-specific risk scoring systems exist, most patients who present to emergency departments with FN are admitted; thus, the potential cost savings for outpatient management are minimal as outpatient management is not yet common practice [36, 37]. Future models could consider patient-specific risk factors. Next, for BC, a TC treatment regimen was assumed [38]. There is some debate in the USA as to whether this regimen is associated with intermediate or high risk of FN [39]. However, in a European setting, the prophylactic strategy commonly employed assumes it is associated with an intermediate risk of FN based on clinical studies reporting an associated FN risk of 15.8% [40], and, as such, that was how it was incorporated into this model. In the previous USA study [15], patients with BC received a taxane (docetaxel) regimen, with an associated baseline FN risk of 16% [41], an intermediate-risk regimen.
Our study reports data for only three countries, confirming a consistent impact of biosimilars across a diversity of European health systems, but it is important to note that the results of this model reflect current practice managing these patient populations only in the countries we have included (France, Germany, and Austria). For these results and potential cost savings to be applicable to other countries, the model would have to be recalculated. For example, published literature in the UK [42] and Australia [43] have reported successful management of patients with BC and low-risk FN in an ambulatory setting and, in the study in Australia, cost savings with this approach. Management of patients in an ambulatory setting would likely result in a lower estimate of cost savings than in our present model. By publishing the details of our model, the cost-effectiveness of expanding reimbursement to cover intermediate-risk chemotherapy regimens can be estimated using HTAs for other health systems, including systems for which transparent costs are not publicly available.
In the absence of data for each cancer type, some inputs were based on studies that examined patients with different cancer types, including the RR of an FN event where it was assumed that the RR for NHL and NSCLC was the same as for BC and self-administration of G-CSF was assumed to be the same across all cancers in the absence of cancer-specific data. Adverse events associated with G-CSFs were not included in the model. The most common adverse event of G-CSFs is bone pain, followed by headache, nausea/vomiting, fever/chills, fatigue, skin reaction, and myalgias, all of which are managed with inexpensive medications that would not add significant costs to the model [44,45,46,47]. Utility values in this model were based on data obtained from clinicians, as direct patient utility data for FN were not available. Finally, the model assumed that all FN events required a hospital admission. FN-related events that occurred outside of the hospital setting were not captured.
The comparability of our findings across three such different health systems suggests that to stay relevant, all HTAs and their associated value-based guidelines will need to undergo regular re-assessments after biosimilars have been launched. The European Commission plans to harmonise HTA methodology in a few years. This suggests that such a rolling programme of post-biosimilar assessment could quickly become routine as a shared resource across a wide range of guideline groups and different national health systems.
Conclusion
Based on this analysis, using biosimilar filgrastim and pegfilgrastim as PP is a cost-effective approach to avoid FN events across all three cancer types and for all three countries, with NSCLC being the most cost-effective. This information suggests there is no rationale to justify additional restrictions to treatment guidelines which exist in some European countries with high costs.
References
Bennett CL, Djulbegovic B, Norris LB, Armitage JO (2013) Colony-stimulating factors for febrile neutropenia during cancer therapy. N Engl J Med 368(12):1131–1139
Culakova E, Thota R, Poniewierski MS, Kuderer NM, Wogu AF, Dale DC et al (2014) Patterns of chemotherapy-associated toxicity and supportive care in US oncology practice: a nationwide prospective cohort study. Cancer Med 3(2):434–444
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106(10):2258–2266
Weycker D, Barron R, Edelsberg J, Kartashov A, Legg J, Glass AG (2014) Risk and consequences of chemotherapy-induced neutropenic complications in patients receiving daily filgrastim: the importance of duration of prophylaxis. BMC Health Serv Res 14:189
Lyman GH, Dale DC, Culakova E, Poniewierski MS, Wolff DA, Kuderer NM et al (2013) The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol 24(10):2475–2484
Tai E, Guy GP, Dunbar A, Richardson LC (2017) Cost of Cancer-Related Neutropenia or Fever Hospitalizations, United States, 2012. J Oncol Pract 13(6):e552–ee61
Lyman GH, Yau L, Nakov R, Krendyukov A (2018) Overall survival and risk of second malignancies with cancer chemotherapy and G-CSF support. Ann Oncol 29(9):1903–1910
Cornes P, Krendyukov A (2019) The evolution of value with filgrastim in oncology. Future Oncol 15(13):1525–1533
Cornes P, Gascon P, Chan S, Hameed K, Mitchell CR, Field P et al (2018) Systematic review and meta-analysis of short- versus long-acting granulocyte colony-stimulating factors for reduction of chemotherapy-induced febrile neutropenia. Adv Ther 35(11):1816–1829
Cornes P, Gascon P, Vulto AG, Aapro M (2020) Biosimilar pegfilgrastim: improving access and optimising practice to supportive care that enables cure. BioDrugs. 34(3):255–263
Klastersky J, de Naurois J, Rolston K, Rapoport B, Maschmeyer G, Aapro M et al (2016) Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol 27(suppl 5):v111–v1v8
Fust K, Li X, Maschio M, Villa G, Parthan A, Barron R et al (2017) Cost-effectiveness analysis of prophylaxis treatment strategies to reduce the incidence of febrile neutropenia in patients with early-stage breast cancer or non-Hodgkin lymphoma. Pharmacoeconomics 35(4):425–438
Hill G, Barron R, Fust K, Skornicki ME, Taylor DC, Weinstein MC et al (2014) Primary vs secondary prophylaxis with pegfilgrastim for the reduction of febrile neutropenia risk in patients receiving chemotherapy for non-Hodgkin’s lymphoma: cost-effectiveness analyses. J Med Econ 17(1):32–42
Wang XJ, Tang T, Farid M, Quek R, Tao M, Lim ST et al (2016) Routine primary prophylaxis for febrile neutropenia with biosimilar granulocyte colony-stimulating factor (nivestim) or pegfilgrastim is cost effective in non-Hodgkin lymphoma patients undergoing curative-intent R-CHOP chemotherapy. PLoS One 11(2):e0148901
Li E, Mezzio DJ, Campbell D, Campbell K, Lyman GH (2021) Primary prophylaxis with biosimilar filgrastim for patients at intermediate risk for febrile neutropenia: a cost-effectiveness analysis. JCO Oncol Pract 17(8):e1235–e1e45
SACO (2002) Health care systems in the Eu a comparative study. In: European Parliament Directorate general for research working paper. https://www.europarl.europa.eu/workingpapers/saco/pdf/101_en.pdf. Accessed 3 Dec 2021
Crawford JWD (2004) First cycle risk of severe and febrile neutropenia in cancer patients receiving systemic chemotherapy: results from a prospective nationwide study. Blood 104(11):2210
Narod SA, Giannakeas V, Sopik V (2018) Time to death in breast cancer patients as an indicator of treatment response. Breast Cancer Res Treat 172(3):659–669
Taylor R, Davis P, Boyages J (2003) Long-term survival of women with breast cancer in New South Wales. Eur J Cancer 39(2):215–222
McDougall JA, Furnback WE, Wang BCM, Mahlich J (2020) Understanding the global measurement of willingness to pay in health. J Mark Access Health Policy 8(1):1717030
Wang L, Baser O, Kutikova L, Page JH, Barron R (2015) The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 23(11):3131–3140
Bezjak A, Lee CW, Ding K, Brundage M, Winton T, Graham B et al (2008) Quality-of-life outcomes for adjuvant chemotherapy in early-stage non-small-cell lung cancer: results from a randomized trial, JBR.10. J Clin Oncol 26(31):5052–5059
Camara RJA, Schwentner L, Friedl TWP, Deniz M, Fink V, Lato K et al (2019) Quality of life during and after adjuvant anthracycline-taxane-based chemotherapy with or without Gemcitabine in high-risk early breast cancer: results of the SUCCESS A trial. Breast Cancer Res Treat 175(3):627–635
Whyte S, Cooper KL, Stevenson MD, Madan J, Akehurst R (2011) Cost-effectiveness of granulocyte colony-stimulating factor prophylaxis for febrile neutropenia in breast cancer in the United Kingdom. Value Health 14(4):465–474
European Medicines Agency (2023) Medicines search. https://www.ema.europa.eu/en/medicines. Accessed 1 May 2022
Becker PS, Griffiths EA, Alwan LM, Bachiashvili K, Brown A, Cool R et al (2020) NCCN Guidelines insights: hematopoietic growth factors, Version 1.2020. J Natl Compr Cancer Netw 18(1):12–22
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ et al (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 33(28):3199–3212
Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L et al (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 24(19):3187–3205
Aapro M, Lyman GH, Bokemeyer C, Rapoport BL, Mathieson N, Koptelova N et al (2021) Supportive care in patients with cancer during the COVID-19 pandemic. ESMO Open 6(1):100038
IQVIA (2021) The impact of biosimilar competition in Europe 2020. https://www.iqvia.com/library/white-papers/the-impact-of-biosimilar-competition-in-europe. Accessed 3 Dec 2021
Amgen (2021) Biosimilar Trends Report, 8th ed. https://www.amgenbiosimilars.com/-/media/Themes/Amgen/amgenbiosimilars-com/Amgenbiosimilars-com/pdf/USA-CBU-80961_Amgen-Biosimilars-Trend-Report.pdf. Accessed 3 Dec 2021
IQVIA (2021) The impact of biosimilar competition in Europe. https://www.iqvia.com/-/media/iqvia/pdfs/library/white-papers/the-impact-of-biosimilar-competition-in-europe-2021.pdf?_=1640100592119. Accessed 3 Dec 2021
Link H, Kerkmann M, Holtmann L, Ortner P (2019) G-CSF guideline adherence in Germany, an update with a retrospective and representative sample survey. Support Care Cancer 27(4):1459–1469
Link H, Nietsch J, Kerkmann M, Ortner P (2016) Adherence to granulocyte-colony stimulating factor (G-CSF) guidelines to reduce the incidence of febrile neutropenia after chemotherapy--a representative sample survey in Germany. Support Care Cancer 24(1):367–376
Dutta B, Huys I, Vulto AG, Simoens S (2020) Identifying key benefits in European off-patent biologics and biosimilar markets: it is not only about price! BioDrugs 34(2):159–170
Baugh CW, Faridi MK, Mueller EL, Camargo CA Jr, Pallin DJ (2019) Near-universal hospitalization of US emergency department patients with cancer and febrile neutropenia. PLoS One 14(5):e0216835
Boccia R, Glaspy J, Crawford J, Aapro M (2022) Chemotherapy-induced neutropenia and febrile neutropenia in the US: a beast of burden that needs to be tamed? Oncologist. 27(8):625–636
de Gregorio A, Janni W, Friedl TWP, Nitz U, Rack B, Schneeweiss A et al (2022) The impact of anthracyclines in intermediate and high-risk HER2-negative early breast cancer-a pooled analysis of the randomised clinical trials PlanB and SUCCESS C. Br J Cancer 126(12):1715–1724
Do T, Medhekar R, Bhat R, Chen H, Niravath P, Trivedi MV (2015) The risk of febrile neutropenia and need for G-CSF primary prophylaxis with the docetaxel and cyclophosphamide regimen in early-stage breast cancer patients: a meta-analysis. Breast Cancer Res Treat 153(3):591–597
Clemons M, Fergusson D, Joy AA, Thavorn K, Meza-Junco J, Hiller JP et al (2021) A multi-centre study comparing granulocyte-colony stimulating factors to antibiotics for primary prophylaxis of docetaxel-cyclophosphamide induced febrile neutropenia. Breast 58:42–49
Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T et al (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358(16):1663–1671
Marshall W, Campbell G, Knight T, Al-Sayed T, Cooksley T (2020) Emergency ambulatory management of low-risk febrile neutropenia: multinational association for supportive care in cancer fits-real-world experience from a UK Cancer Center. J Emerg Med 58(3):444–448
Teh BW, Brown C, Joyce T, Worth LJ, Slavin MA, Thursky KA (2018) Safety and cost benefit of an ambulatory program for patients with low-risk neutropenic fever at an Australian centre. Support Care Cancer 26(3):997–1003
Blayney DW, Schwartzberg L (2022) Chemotherapy-induced neutropenia and emerging agents for prevention and treatment: a review. Cancer Treat Rev 109:102427
Sandoz GmbH (2013) Zarzio SmPC. https://www.ema.europa.eu/en/documents/product-information/zarzio-epar-product-information_en.pdf. Accessed 3 Dec 2021
Sandoz GmbH (2018) Ziextenzo SmPC. https://www.ema.europa.eu/en/documents/product-information/ziextenzo-epar-product-information_en.pdf. Accessed 3 Dec 2021
Lapidari P, Vaz-Luis I, Di Meglio A (2021) Side effects of using granulocyte-colony stimulating factors as prophylaxis of febrile neutropenia in cancer patients: a systematic review. Crit Rev Oncol Hematol 157:103193
Funding
This work was funded by Sandoz, Inc.
Author information
Authors and Affiliations
Contributions
SC, NK, and AM developed and validated the model. BKS and SC wrote the main manuscript text with support from SH and AM. MA, PC, HL, MDP, and RT provided country-specific expertise for the model and manuscript text. All authors reviewed the manuscript and approved for submission.
Corresponding author
Ethics declarations
Ethics approval
No ethics approval was required for this work as it did not involve patient-specific information.
Competing interests
SC and BKS are employees of Xcenda. SH, AM, and NK are employees of Sandoz. RT and MA have no competing interests to disclose. HL reports financial interests via advisory board involvement in the following: Apogepha, G1 Therapeutics, Hogg Robinson Germany, Lindis Blood Care, Pharmacosmos, Servier Deutschland GmbH, SIGAL SMS GmbH, Takeda, and Teva; expert testimony for Samsung Bioepis and Ethics Committee at the Rhineland-Palatinate Medical Association; invited speaker for Teva and Vision Plus Mailand; and ownership interest, research grants, licencing fees, coordinating PI, and steering committee member for Onkodin GmbH, AMGEN and Pharmacosmos, Viatris, and Teva-Xcenda, respectively. PC declares honoraria or support for attending meetings from Accord Healthcare, Amgen, Astro Pharma, European Association for Hospital Pharmacists, European Commission, Medicines for Europe/European Generics Association, Medscape, Mylan, Napp, Pfizer/Hospira, Sandoz, Teva and Viatris. MP reports clinical research projects with Bayer, Sandoz, Pierre Fabre, Fresenius, Astellas, Janssen, and Sanofi; Speakers’ bureau with Amgen, KyowaKirin, MSD, and Mundipharma; Honoraria from Novartis and AstraZeneca; travel and accommodations and expenses from Pfizer and Novartis.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aapro, M.S., Chaplin, S., Cornes, P. et al. Cost-effectiveness of granulocyte colony-stimulating factors (G-CSFs) for the prevention of febrile neutropenia (FN) in patients with cancer. Support Care Cancer 31, 581 (2023). https://doi.org/10.1007/s00520-023-08043-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08043-4