Abstract
Purpose
Tumour blood vessels are structurally and functionally abnormal, resulting in areas of hypoxia and heterogeneous blood supply. Aerobic exercise may modulate tumour blood flow and normalise the tumour microenvironment to improve chemotherapy delivery. This systematic review and meta-analysis aimed to evaluate the effect of the aerobic exercise mode on tumour hypoxia, vascularisation and blood flow.
Methods
Four online databases were searched. Preclinical and clinical randomised controlled trials examining the effects of aerobic exercise training on hypoxia, vascularisation or blood flow in solid tumours were included. The risk of bias was assessed and a meta-analysis performed.
Results
Seventeen preclinical studies and one clinical study met criteria. Eleven studies assessed hypoxia, 15 studies assessed vascularisation and seven evaluated blood flow. There was large variability in measurement methods, tumour types and exercise program designs. The overall risk of bias was unclear in clinical and preclinical studies, owing to poor reporting. There was no significant effect of aerobic exercise on hypoxia (SMD = −0.17; 95% CI = −0.62, 0.28; I2 = 60%), vascularisation (SMD = 0.07; 95% CI = −0.40, 0.55; I2 = 71%) or blood flow (SMD = 0.01; 95% CI = −0.59, 0.61; I2 = 63%).
Conclusion
There is heterogeneity in methodology, resulting in evidence that is inconsistent and inconclusive for the effects of aerobic exercise on hypoxia, vascularisation and blood flow. Most evidence of aerobic exercise effects on tumour blood flow is in animal models, with very limited evidence in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer incidence worldwide in 2016 was 17.2 million cases, with a 28% increase observed between 2006 and 2016 [1]. There are many modifiable risk factors for cancer development, including obesity and physical inactivity, which combined contribute to 25% of cancer incidence [2, 3]. Exercise during cancer treatment is recognised as an important adjunct to cancer treatment, with significant benefits on quality of life and a reduction in treatment side effects [4,5,6,7]. It has been proposed that exercise may also induce adaptations to tumour vasculature and thus stimulate benefit to clinical outcomes of cancer treatment [8].
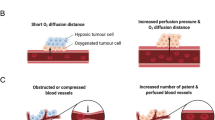
Unlike in healthy tissues, where blood vessels typically run in parallel, blood vessels in tumours have an unstructured distribution. Tumour centres have high interstitial fluid pressure, leading to the collapse and abnormal compression of vasculature [8]. These structural and functional abnormalities lead to heterogeneous blood flow in in regions of the tumour which is further compounded by necrotic tumour centres [8]. The implication of these abnormal features results in a hypoxic tumour environment that is proposed to suppress immune function, limit the transport of immune cells to tumour regions and increase metastasis, leading to poorer patient prognosis [8,9,10,11].
Acute aerobic exercise increases total blood flow to healthy active tissue (e.g. contracting skeletal muscle) through the combination of increased cardiac output, increased blood pressure and local vessel vasodilation, with vasoconstriction reducing or maintaining blood flow to inactive tissues [8, 12]. Arterioles in tumours are poorly developed and lack functional smooth muscle [13] and subsequently have a reduced myogenic vasoconstriction response at high pressures such as during aerobic exercise [14]. This inability to vasoconstrict suggests that exercise-induced increased cardiac output, in addition to increased blood pressure, would drive an increase in tumour perfusion through increased total blood flow [14].
Repeated bouts of aerobic exercise cause vascular adaptions in healthy tissue. The combination of angiogenesis and decreased resistance enables increased blood flow to active healthy tissue [12]. These effects have been proposed to be paralleled in tumours, such that aerobic exercise training may cause adaptations that modulate tumour blood flow through increased blood vessel density and improved organisation and vessel function [8, 13]. If these adaptions occur, aerobic exercise training may normalise the tumour microenvironment and facilitate increased blood flow and reduced hypoxia, which may have benefits for reducing cancer progression. Given that many cancer treatments such as chemotherapy are administered intravenously, the aerobic exercise training effects of increased blood flow and improved vessel function have the additional potential for improving the delivery and therefore efficacy of such treatments [14].
The purpose of this systematic review is to: (i) summarise the effects of aerobic exercise training on tumour hypoxia, vascularisation and blood flow; and (ii) evaluate the methodological rigour of this literature.
Methods
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. This review was registered with the PROSPERO Register of Systematic Reviews (CRD42020159201).
Eligibility criteria
Trials were included if they: (1) were a peer-reviewed journal full-text article in English; (2) used a randomised or quasi-randomised study design with a control group; (3) involved humans or animals with a solid malignant tumour; (4) the intervention group performed repeated bouts of any type of aerobic exercise of ≥2 sessions; (5) the control group performed no structured or unstructured aerobic exercise; (6) measured hypoxia, vascularisation, blood flow or indicators thereof.
Search strategy and study selection
We searched Medline via OvidSP (1946–present), EMBASE via OvidSP (1947–present), Scopus (all years) and CINAHL Complete (all years) in September 2021 using the following search terms: ‘exercise’ OR ‘physical activity’ OR ‘exercise therapy’ OR ‘aerobic exercise’ AND ‘neoplasm’ OR ‘tumour’ OR ‘tumor’ OR ‘carcinoma’ OR ‘cancer’ OR ‘tumour microenvironment’ OR ‘tumor microenvironment’ OR ‘tumour vasculature’ OR ‘tumor vasculature’ AND ‘blood delivery’ OR ‘blood flow’ OR ‘vascularisation’ OR ‘vascular function’ OR ‘vascular remodeling’ OR ‘hypoxia’ OR ‘tumor hypoxia’ OR ‘tumour hypoxia’ OR ‘oxygenation’.
Two reviewers (CSL, JY) independently performed the initial screening by title and abstract based on the eligibility criteria. Full-text versions of potential eligible studies were then assessed by two reviewers independently (CSL, JY). A third reviewer (KE) screened full-text studies if there were disagreements between the researchers regarding eligibility.
Outcome measures and data extraction
Two reviewers (CSL, LR) independently extracted data, and a third reviewer (KE) performed data extraction if there were disagreements between the researchers. Data were extracted for the three variables; hypoxia, vascularisation and blood flow. Hypoxia included any measure indicative of hypoxia as identified by the study author. Vascularisation includes microvessel density and changes in vessel physiology (including number of functional and patent vessels). Blood flow included changes in both tumour vessel perfusion and tissue perfusion (by MRI or Hoechst 33342 staining). Data extracted were recorded as the change in mean values from baseline for each group, and the standard error of the mean (SMD), standard deviation (SD) or confidence interval (CI) was also recorded. Study characteristics extracted included study details and design, recruitment source and method, participant results, experimental protocol, adherence and funding.
Quality assessment
The included studies were assessed for internal validity using SYRCLE’s Risk of Bias Tool [15] or Cochrane Risk of Bias Tool [16]. The SYRCLE Risk of Bias Tool is used to determine the internal validity of animal studies. The tool contains 10 questions relating to 6 different domains of bias; selection bias, performance bias, detection bias, attrition bias, reporting bias and other biases. Each entry was scored as ‘no’ (indicating high risk), ‘yes’ (indicating low risk) or ‘unclear’ (indicating unclear risk). Each study was assessed in its entirety, irrespective of whether the study had multiple outcome measures. Baseline characteristics included age, sex, tumour type, site of tumour injection and timing of tumour induction prior to randomisation. Studies had to explicitly report study characteristics to assess the risk of bias. Housing allocation was also assessed in Domain 10, specifically if animals were housed individually and analysed individually.
The Cochrane Risk of Bias Tool is used to determine the internal validity of human studies [16]. The tool contains 5 questions that each covers a domain of bias; selection bias, performance bias, detection bias, attrition bias and reporting bias. Within each domain are signalling questions that draw relevant information from the study to determine risk of bias. The responses to the signalling questions are formulated in an algorithm to determine a judgement of ‘low risk’, ‘some concern’ or ‘high risk’. The Risk of Bias Tool was used for each outcome measure if the study included multiple outcome measures.
Two researchers (CSL, LR) independently performed these assessments. A third reviewer (JY) performed an assessment if there were disagreements between the researchers.
Statistical analysis
A meta-analysis was performed for hypoxia, vascularisation and blood flow. Data from the outcome measures and indicators thereof were extracted as the mean and standard deviation. Data that were presented as SEM or CI were converted to SD. Data were included if they were quantitative and physiologically plausible. Relative values and fold changes were excluded if raw data were unable to be obtained. Negative values, such as for changes in hypoxia-inducible factor 1-alpha (HIF1α), were excluded as these are not physiologically feasible. For studies that had a range in sample size, the lower end of the sample size was used to avoid overpowering the study. The raw data can be found in the Supplemental materials (Supplemental Table 1).
The meta-analysis was performed in RStudio (R version 4.0.3) using a random-effects model. Heterogeneity was assessed by I2, and a subgroup analysis was performed on hypoxia, vascularisation and blood flow for animals, species, aerobic exercise mode, tumour location and study duration. The meta-analysis was controlled for the inclusion of multiple datasets that were compared to the same control group within a single study using the following equation; Ncorrected control = Ncontrol / number of experimental groups [17].
Results
Study selection
The initial database searches yielded 2692 studies (Fig. 1). After duplicates were removed, a total of 1680 studies were screened by title and abstract. Thirty-nine full-text articles proceeded for further review. A total of 18 studies were deemed suitable for inclusion in this review.
Study and participant characteristics
Details of study and participant characteristics of the included studies are presented in Table 1. Of the 18 studies included, 17 were preclinical and one was clinical. Only one study was published prior to 2010. Multiple studies assessed more than one outcome measure; 11 assessed hypoxia, 15 assessed vascularisation and seven evaluated blood flow. Of the 17 preclinical studies, 14 used mice and four used rats. Study duration ranged from five to 245 days. Aerobic exercise in the preclinical studies included both voluntary (n = 6) and forced exercise (n = 11). The six studies that used voluntary aerobic exercise adopted wheel running, performed daily or every second day. The remaining 11 studies used treadmill running, of which nine studies were five times per week, two studies were daily and one study was every second day. One study used two different aerobic exercise frequencies in different groups. One clinical study explored cycle training sessions three times per week at 55–100% VO2peak in women with breast cancer.
Risk of bias
Preclinical: SYRCLE Risk of Bias
The risk of bias across the preclinical studies is reported in Fig. 2. Of concern, more than half of the included studies (n = 11) had a high risk of attrition bias due to not reporting missing data appropriately. However, all 17 studies were free of selective outcome reporting as all available results were presented as described in the study methods. Nine studies reported clear baseline characteristics and therefore were assessed as having a low risk, while the remaining eight studies did not clearly report all baseline characteristics and were assessed as having an unclear risk. Most other outcomes were assessed as having an unclear risk due to poor reporting of study methodology.
Clinical: Cochrane Risk of Bias
Overall, there was a high risk of bias for the clinical study, primarily a result of missing outcome data for hypoxia and vascularisation (Fig. 3). There were additional concerns regarding randomisation, as the study did not detail the concealment procedure. Blinding of participants was not possible due to the pragmatic nature of the exercise intervention, although there was still low risk of bias for deviations from the intended intervention. Although the overall risk of bias was high, there was low risk of bias in the measurement and reporting of all outcomes (Fig. 3).
Hypoxia
Measures of hypoxia included HIF1α (n = 6), EF5 (n = 2), pimonidazole (n = 2), carbonic anhydrase IX (CAIX) (n = 2) and partial pressure of oxygen (PO2) (n = 1) (Table 2). Some studies assessed multiple measures of hypoxia. Of the 11 studies which assessed hypoxia, six studies reported a decrease in hypoxia [18,19,20,21,22,23], one study reported an increase [24] and five studies reported no change [23, 25,26,27,28]. One study [23] utilised four different datasets, inclusive of two different tumour types and two different measurement methods, finding a decrease and no change in hypoxia within the one paper. Six studies were included in the meta-analysis for hypoxia [18, 20, 23, 25, 26, 28], with three studies having multiple measures analysed [23, 25, 26]. Four studies were excluded from the meta-analysis due to implausible physiological values or lack of continuous mean data [19, 22, 24]. Overall, there was no significant effect of aerobic exercise on hypoxia (SMD = −0.17; 95% CI = −0.62, 0.28; I2 = 60%) (Fig. 4a).
a Meta-analysis of preclinical studies investigating the effect of exercise on hypoxia. SD, standard deviation; SMD, standardised mean difference; 95% CI, 95% confidence interval (upper; lower limits). b Meta-analysis of preclinical studies investigating the effect of exercise on vascularisation. SD, standard deviation; SMD, standardised mean difference; 95% CI, 95% confidence interval (upper; lower limits). c Meta-analysis of preclinical studies investigating the effect of exercise on blood flow. SD, standard deviation; SMD, standardised mean difference; 95% CI, 95% confidence interval (upper; lower limits)
Vascularisation
Measures of vascularisation included microvessel density (n = 14), functional vessels (n = 2), vessel length (n = 1), patent vessels (n = 1) and indicators for vessel function and structure (n = 3) (Table 2). Four studies reported an increase in microvessel density [18, 24, 29, 30], three studies reported a decrease in microvessel density [19, 31, 32] and eight studies reported no change in microvessel density [20, 23, 25,26,27,28, 33, 34]. Twelve studies were included in the meta-analysis for vascularisation [18,19,20, 23,24,25,26,27,28,29,30, 33, 34], with four studies having multiple measures [23, 25, 26, 34]. One study was excluded due to no baseline data [32]. One study [31] was excluded from the presented forest plot for vascularisation due to being an outlier, resulting in reduced legibility of the remainder of the meta-analysis results (SMD = 0.07; 95% CI = −0.51, 0.64; I2 = 79%) (full plot available in Supplemental Figure 1). The exclusion made no difference in the overall effect size. Overall, there was no effect of aerobic exercise on vascularisation (SMD = 0.07; 95% CI = −0.40, 0.55; I2 = 71%) (Figure 4b).
Blood flow
Of the seven studies that evaluated blood flow, three reported an increase [18, 24, 28], one reported a decrease [27] and three reported no change [25, 26, 35] (Table 2). Four studies were included in the meta-analysis for blood flow [25,26,27,28, 35], with two studies having multiple measures, including blood flow and perfusion [25, 26]. One study was excluded from the meta-analysis due to no quantitative data reported, and another study was excluded as there were no mean data [18, 24]. Overall, there was no significant effect of aerobic exercise on blood flow (SMD = 0.01; 95% CI = −0.59, 0.61; I2 = 63%) (Figure 4c).
Sub-group analysis
Given the heterogeneity of methodology in the studies included in this review, the total meta-analysis is limited. In an attempt to review the data taking into account some of these methodological differences, we performed sub-group analyses for animals, species, aerobic exercise mode, tumour location and study duration. However, it should be noted that these sub-groups included large heterogeneity, such as in exercise mode (for example, treadmill exercise dose). We therefore performed sub-group analyses using broad categories to analyse the data. The results from the sub-analyses showed no significant effect in any of the analyses. Sub-group analyses can be found in the Supplemental materials (Supplemental Figures 2a–e, 3a–e and 4a–e).
Discussion
The findings from this systematic review and meta-analysis demonstrate that among 17 preclinical studies and one clinical study, aerobic exercise training had no significant effect on tumour hypoxia, vascularisation or blood flow. To our knowledge, this is the only systematic review exploring the effects of hypoxia, vascularisation and blood flow on all three of these related outcomes. Concerningly, there was a high risk for attrition bias in the preclinical studies, and most other domains of bias were of unclear risk. Of the 18 studies included in this review, 17 were published within the last decade, suggesting that there is a growing interest in this area. Although the preclinical body of evidence is growing, the synthesis of data is limited by the variability in animal type and study methodology. The measurement of the outcomes reported herein is possible in a human population, as evidenced in Jones et al. (2013). Therefore, future knowledge gains may be made by exploring these effects in humans for translation to clinical practice.
We anticipated that aerobic exercise would drive an association between our outcome measures; that aerobic exercise training would result in an increase in vascularisation that would cause increased blood flow and consequently decreased hypoxia, representing beneficial changes in the tumour microenvironment with the potential to reduce tumour growth and improve patient prognosis. This assumption has been based on the literature, exemplified by recent narrative reviews [36, 37]. However, the expected pattern of change was not found in this review, with studies reporting aerobic exercise-induced decreases and increases in all outcome measures and combinations of decreases and increases that do not support our assumption. Although our findings contrast with recent narrative reviews, our conclusions are supported by the systematic inclusion of all available data.
In the period between registration and completion of this review, Esteves et al. [38] published a systematic review and meta-analysis that included one of the outcomes we assessed: vascularisation. The authors found an effect of aerobic exercise on vascularisation, which is in conflict with the findings from this review. We have described a number of concerns with the methodology, data extraction and data analysis of their review elsewhere [39]. The current comprehensive review and meta-analysis uses a more appropriate statistical analysis recommended for preclinical studies [40,41,42] and includes all available studies, providing a more accurate evaluation of the current literature.
Contrary to our expectations, we found that there were inconsistent findings for all explored outcomes. We expected to see decreases in hypoxia; however, of the 11 studies which assessed hypoxia, one [24] found an unexpected increase and five [23, 25,26,27,28] found no change. We expected increased vascularisation; however, three of 15 studies [19, 31, 32] showed a reduction and eight [20, 23, 25,26,27,28, 33, 34] showed no change. Our expectation of increased blood delivery was also not met; three studies demonstrated no change [25, 26, 35], and one study demonstrated a decrease [27] in blood flow. These unexpected results in hypoxia, vascularisation and blood flow may be a result of many factors, including experimental design differences as well as intra-tumoural factors. For example, physiological mechanisms that may have contributed to these results include angiogenesis development and/or decreased inflammatory immune cells. Aerobic exercise induces the formation of new blood vessels through regulatory factors such as increases in VEGF, which promotes angiogenesis [24]. Although angiogenesis may increase microvessel density and subsequent blood flow to tumours, the dysfunction in tumour blood vessels (specifically leaky blood vessels and low permeability) may mean that this is not translated to changes in hypoxia if oxygen diffusion is poor [9, 10, 31, 40]. Furthermore, tumour blood vessels are heterogeneous in distribution, leading to inconsistent oxygen supply [8], which may mean areas of increased hypoxia within the tumour despite potential increases in overall blood flow through increased microvessel density. Therefore, a single hypoxia measurement may not represent the level of hypoxia elsewhere in the tumour. Immune cell’s contribution to angiogenesis has also been suggested to be altered by aerobic exercise training. For example, inflammatory macrophages in tumours have been shown to have reduced activation after aerobic training [32]. This type of reduced inflammatory activation may downregulate angiogenesis and reduced microvessel density, resulting in less blood delivery and increased hypoxia [32].
Variability in study methodology and quality can create significant bias in the results. Features of poor study methodology, such as lack of reporting, heterogeneity of baseline characteristics and external influences such as housing (in preclinical research), aerobic exercise outside of the intervention and significant missing data, can largely impact results both within studies and when comparing studies. The risk of bias results from this review should be used to guide future preclinical and clinical methodology to better compare outcome measures across studies. Housing was poorly reported, which is important for social stress effects [43]. Additionally, the impact of stress on animals, such as stimulation for forced aerobic exercise, is particularly significant in cancer outcomes, whereby it has been postulated that the immune response plays a part in the development of tumour vasculature to promote microvessel density and tumour growth in mice [44, 45]. The characteristics of aerobic exercise were poorly reported across studies. Six studies [18, 22, 24,25,26, 28] used voluntary wheel running, which suffers from a lack of control of intensity and, therefore, dose. Seven papers included a familiarisation period of aerobic exercise for an average of 18 days [18,19,20, 29, 32, 33, 35], whereby subjects would perform additional aerobic exercise after tumour induction but prior to the aerobic exercise intervention. Exercise duration includes a combination of familiarisation plus intervention, which further complicates the dose of aerobic exercise delivered, particularly in preclinical models. In the current analysis, we saw heterogeneity in animal type and species (14 mice studies with five different species and four rat studies with three different species), making comparison of results between papers problematic and suitability for human models debateable [46].
Tumour type and location likely play a considerable role in determining the effects of aerobic exercise on blood flow. Host tissue is anatomically and functionally different, and thus features of the tumour microenvironment that influence blood flow may also differ based on tumour location. Therefore, it may not be equal to compare tumour type and location. For example, tumours located in host tissue that has a high blood supply at rest, such as the brain, are more likely to use vascular co-option, which encourages greater tumour vascularisation [47]. Furthermore, vasculogenic mimicry occurs in some tumour types, such as melanoma and breast, and can also increase blood supply to and within the tumour [48]. In preclinical models, tumours are induced orthotopically (tumours that are injected into the corresponding host tissue) or ectopically (tumours injected subcutaneously into a different host tissue). Garcia and colleagues [49] directly compared ectopic prostate tumours placed subcutaneously with orthotopic prostate tumours and found increased blood flow in orthotopic tumours after aerobic exercise, suggesting that tumour location plays an important role irrespective of tumour type. We included 13 papers that investigated orthotopically injected tumours and two papers that investigated subcutaneously injected tumours, and we observed no difference in blood flow after aerobic exercise in orthotopic compared to subcutaneous tumours, which is contrary to the results of Garcia et al [49]. However, a majority of the papers in this systematic review investigated breast tumours, whilst Garcia et al. [49] investigated prostate tumours. Therefore, further research needs to be conducted to determine if tumour type, in addition to tumour location, influences blood flow.
Whilst our findings do not favour aerobic exercise training in terms of tumour hypoxia, vascularisation and blood flow, several systematic reviews and meta-analyses have found beneficial effects of aerobic exercise training for tumour growth and development [44, 50]. Given this contrast, we briefly examined tumour growth outcomes, which were reported in 13 studies included in this review [18, 21, 22, 24,25,26, 28,29,30,31,32,33,34]. Of these, there were inconsistent results in tumour growth; one reported increased growth, four showed decreased growth and nine showed no change in tumour size. One study [34] showed both a decrease in one tumour type and an increase in another tumour type. We observed similar inconsistent patterns of change in hypoxia, vascularisation and blood flow even with studies showing the same tumour growth changes. However, it should be noted that tumour growth was not a primary outcome of this review and the reported findings are descriptive only. As the studies included in this review did not mirror the results from the two published systematic reviews on tumour growth, we believe that the mechanisms of action for aerobic exercise on tumour growth are complex and not well described by this literature. It is possible that the mechanisms of aerobic exercise effects could involve interactions between hypoxia, vascularisation and blood flow; however, more consistent data, methodology and reporting are necessary to further explore these associations. It is also likely that other systemic or intra-tumoural factors, such as inflammation, immune responses, myokine signalling and endocrine responses and adaptations, impact tumour growth [44, 51, 52].
Limitations
Despite using author-reported results, we found that there were two instances where there were differences in the significance of findings between author-reported results and the meta-analysis calculation. Our careful review and meta-analysis showed inconsistencies within a paper, which were confirmed with the authors but may have been the cause of the different outcomes in the analysis. Subsequently, it is possible that the conclusions of this review would differ without the inclusion of a meta-analysis calculation. Another limitation is that although we conducted sub-group analyses, the results were limited due to poor reporting and heterogeneous study design. Furthermore, our meta-analysis examined one mode of exercise, aerobic exercise only, which does not fully represent the effect of all exercise modes, including resistance exercise.
Conclusion
Among the studies included in this systematic review, aerobic exercise training did not have an effect on tumour hypoxia, vascularisation or blood flow. However, there was great methodological heterogeneity, which may have contributed to the inconsistent findings. Future preclinical studies need improved study design and reporting to provide deeper insights into the complex interactions between hypoxia, vascularisation and blood flow. Furthermore, improved reporting and subsequent analysis of aerobic exercise parameters in future studies, specifically intensity and dosage, will give rise to a better understanding of the effects of aerobic exercise on tumour microenviroment for translation to clinical studies. Given the established benefits of aerobic exercise in reducing cancer burden, understanding the mechanisms is an important step towards designing the most efficacious interventions and best practise for translation to clinical studies.
Data availability
The datasets from this review are available from the corresponding author on reasonable request.
References
Global Burden of Disease Cancer, Fitzmaurice C, Akinyemiju TF et al (2018) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 4(11):1553–1568. https://doi.org/10.1001/jamaoncol.2018.2706
Colditz GA, Wei EK (2012) Preventability of cancer: the relative contributions of biologic and social and physical environmental determinants of cancer mortality. Ann Rev Public Health. 33:137–156. https://doi.org/10.1146/annurev-publhealth-031811-124627
de Boer MC, Worner EA, Verlaan D, van Leeuwen PAM (2017) The mechanisms and effects of physical activity on breast cancer. Clin Breast Cancer. 17(4):272–278. https://doi.org/10.1016/j.clbc.2017.01.006
Cave J, Paschalis A, Huang CY et al (2018) A systematic review of the safety and efficacy of aerobic exercise during cytotoxic chemotherapy treatment. Support Care Cancer. 26(10):3337–3351. https://doi.org/10.1007/s00520-018-4295-x
Cormie P, Zopf EM, Zhang X, Schmitz KH (2017) The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol Rev. 39(1):71–92. https://doi.org/10.1093/epirev/mxx007
Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O (2012) Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev (8). 10.1002/14651858.CD008465.pub2
Tomlinson D, Diorio C, Beyene J, Sung L (2014) Effect of exercise on cancer-related fatigue: a meta-analysis. Am J Phys Med Rehabil. 93(8):675–686. https://doi.org/10.1097/PHM.0000000000000083
Wiggins JM, Opoku-Acheampong AB, Baumfalk DR, Siemann DW, Behnke BJ (2018) Exercise and the tumor microenvironment: potential therapeutic implications. Exerc Sport Sci Rev. 46(1):56–64. https://doi.org/10.1249/JES.0000000000000137
Dewhirst MW, Secomb TW (2017) Transport of drugs from blood vessels to tumour tissue. Nat Rev Cancer 17(12):738–750. https://doi.org/10.1038/nrc.2017.93
Jain RK (1990) Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev 9(3):253–266. https://doi.org/10.1007/BF00046364
Kumar V, Gabrilovich DI (2014) Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology. 143(4):512–519. https://doi.org/10.1111/imm.12380
Brooks GA (2005) Exercise physiology: human bioenergetics and its applications, 4th edn. McGraw-Hill, Boston
Siemann DW, Horsman RM (2015) Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol Ther. 153:107–124. https://doi.org/10.1016/j.pharmthera.2015.06.006
McCullough DJ, Stabley JN, Siemann DW, Behnke BJ (2014) Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. J Natl Cancer Inst. 106(4). https://doi.org/10.1093/jnci/dju036
Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 14(1):43. https://doi.org/10.1186/1471-2288-14-43
Higgins JPT, Altman DG, Gøtzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 343. https://doi.org/10.1136/bmj.d5928
Vesterinen HM, Sena ES, Egan KJ et al (2014) Meta-analysis of data from animal studies: a practical guide. J Neurosci Meth. 221:92–102. https://doi.org/10.1016/j.jneumeth.2013.09.010
Betof AS, Lascola CD, Weitzel D et al (2015) Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J Natl Cancer Inst. 107(5). https://doi.org/10.1093/jnci/djv040
Isanejad A, Alizadeh AM, Amani Shalamzari S et al (2016) MicroRNA-206, let-7a and microRNA-21 pathways involved in the anti-angiogenesis effects of the interval exercise training and hormone therapy in breast cancer. Life Sci. 151:30–40. https://doi.org/10.1016/j.lfs.2016.02.090
McCullough DJ, Nguyen LM, Siemann DW, Behnke BJ (2013) Effects of exercise training on tumor hypoxia and vascular function in the rodent preclinical orthotopic prostate cancer model. J Appl Physiol. 115(12):1846–1854. https://doi.org/10.1152/japplphysiol.00949.2013
Rafiei MM, Soltani R, Kordi M, Nouri R, Gaeini A (2021) Gene expression of angiogenesis and apoptotic factors in female BALB/c mice with breast cancer after eight weeks of aerobic training. Iran J Basic Med Sci 24(9):1196–1202. https://doi.org/10.22038/ijbms.2021.55582.12427
Wakefield ZR, Tanaka M, Pampo C et al (2021) Normal tissue and tumor microenvironment adaptations to aerobic exercise enhance doxorubicin anti-tumor efficacy and ameliorate its cardiotoxicity in retired breeder mice. Oncotarget 12(18):1737–1748. https://doi.org/10.18632/oncotarget.28057
Morrell MB, Alvarez-Florez C, Zhang A et al (2019) Vascular modulation through exercise improves chemotherapy efficacy in Ewing sarcoma. Pediatr Blood Cancer. 66(9):e27835. https://doi.org/10.1002/pbc.27835
Jones LW, Antonelli J, Masko EM et al (2012) Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J Appl Physiol. 113(2):263–272. https://doi.org/10.1152/japplphysiol.01575.2011
Buss LA, Ang AD, Hock B, Robinson BA, Currie MJ, Dachs GU (2020) Effect of post-implant exercise on tumour growth rate, perfusion and hypoxia in mice. PLoS ONE. 15(3):e0229290. https://doi.org/10.1371/journal.pone.0229290
Buss LA, Dachs GU (2018) Voluntary exercise slows breast tumor establishment and reduces tumor hypoxia in ApoE−/− mice. J Appl Physiol. 124(4):938–949. https://doi.org/10.1152/japplphysiol.00738.2017
Jones LW, Fels DR, West M et al (2013) Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Prev Res. 6(9):925–937. https://doi.org/10.1158/1940-6207.CAPR-12-0416
Jones LW, Viglianti BL, Tashjian JA et al (2010) Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Appl Physiol. 108(2):343–348. https://doi.org/10.1152/japplphysiol.00424.2009
Faustino-Rocha AI, Silva A, Gabriel J et al (2016) Long-term exercise training as a modulator of mammary cancer vascularization. Biomed Pharmacother. 81:273–280. https://doi.org/10.1016/j.biopha.2016.04.030
Florez Bedoya CA, Cardoso ACF, Parker N et al (2019) Exercise during preoperative therapy increases tumor vascularity in pancreatic tumor patients. Sci Rep. 9(1):13966. https://doi.org/10.1038/s41598-019-49582-3
Saran U, Guarino M, Rodriguez S et al (2018) Anti-tumoral effects of exercise on hepatocellular carcinoma growth. Hepatol Commun. 2(5):607–620. https://doi.org/10.1002/hep4.1159
Zielinski MR, Muenchow M, Wallig MA, Horn PL, Woods JA (2004) Exercise delays allogeneic tumor growth and reduces intratumoral inflammation and vascularization. J Appl Physiol. 96(6):2249–2256. https://doi.org/10.1152/japplphysiol.01210.2003
Dufresne S, Guéritat J, Chiavassa S et al (2020) Exercise training improves radiotherapy efficiency in a murine model of prostate cancer. FASEB J. 34(4):4984–4996. https://doi.org/10.1096/fj.201901728R
Schadler KL, Thomas NJ, Galie PA et al (2016) Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget 7(40):65429–65440. https://doi.org/10.18632/oncotarget.11748
Faustino-Rocha AI, Silva A, Gabriel J et al (2017) Long-term exercise training and mammary tumour vascularization: thermography, ultrasonography and immunohistochemistry. J Comp Pathol. 156(1):119. https://doi.org/10.1016/j.biopha.2016.04.030
Esteves M, Monteiro MP, Duarte JA (2020) Role of regular physical exercise in tumor vasculature: favorable modulator of tumor milieu. Int J Sports Med. https://doi.org/10.1055/a-1308-3476
Schumacher O, Galvão DA, Taaffe DR, Chee R, Spry N, Newton RU (2021) Exercise modulation of tumour perfusion and hypoxia to improve radiotherapy response in prostate cancer. Prostate Cancer Prostatic Dis. 24(1):1–14. https://doi.org/10.1038/s41391-020-0245-z
Esteves M, Monteiro MP, Duarte JA (2021) The effects of physical exercise on tumor vasculature: systematic review and meta-analysis. Int J Sports Med. https://doi.org/10.1055/a-1533-1876
Seet-Lee C, Morahan H, Yee J, Edwards KM. (2022) Letter to the editor on "The effects of physical exercise on tumor vasculature: systematic review and meta-analysis". Int J Sports Med. 43(3):286–287. https://doi.org/10.1055/a-1754-3323
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) (2021) Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane. https://training.cochrane.org/handbook. Accessed 16 Sept 2021
Borenstein M (ed) (2009) Introduction to meta-analysis. John Wiley & Sons, Chichester, U.K
Hooijmans CR, IntHout J, Ritskes-Hoitinga M et al (2014) Meta-analyses of animal studies: an introduction of a valuable instrument to further improve healthcare. ILAR J 55(3):418–426. https://doi.org/10.1093/ilar/ilu042
Bartolomucci A (2007) Social stress, immune functions and disease in rodents. Front Neuroendocrin. 28(1):28–49. https://doi.org/10.1016/j.yfrne.2007.02.001
Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, Jones LW (2016) Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: a critical systematic review of in vivo preclinical data. Cancer Res. 76(14):4032–4050. https://doi.org/10.1158/0008-5472.CAN-16-0887
Thaker PH, Han LY, Kamat AA et al (2006) Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 12(8):939–944. https://doi.org/10.1038/nm1447
Mollard S, Mousseau Y, Baaj Y et al (2011) How can grafted breast cancer models be optimized? Cancer Biol Ther. 12(10):855–864. https://doi.org/10.4161/cbt.12.10.18139
Seano G, Jain RK (2020) Vessel co-option in glioblastoma: emerging insights and opportunities. Angiogenesis. 23(1):9–16. https://doi.org/10.1007/s10456-019-09691-z
Luo Q, Wang J, Zhao W et al (2020) Vasculogenic mimicry in carcinogenesis and clinical applications. J Hematol Oncol Pharm. 13(1):19. https://doi.org/10.1186/s13045-020-00858-6
Garcia E, Becker VG, McCullough DJ et al (2016) Blood flow responses to mild-intensity exercise in ectopic vs. orthotopic prostate tumors; dependence upon host tissue hemodynamics and vascular reactivity. J Appl Physiol 121(1):15–19. https://doi.org/10.1152/japplphysiol.00266.2016
Eschke RC, Lampit A, Schenk A et al (2019) Impact of physical exercise on growth and progression of cancer in rodents—a systematic review and meta-analysis. Front Oncol. 9:35. https://doi.org/10.3389/fonc.2019.00035
Pedersen L, Christensen JF, Hojman P (2015) Effects of exercise on tumor physiology and metabolism. Cancer J 21(2):111–116. https://doi.org/10.1097/PPO.0000000000000096
Kim J, Galvao DA, Newton RU, Gray E, Taaffe DR (2021) Exercise-induced myokines and their effect on prostate cancer. Nat Rev Urol. 18(9):519–542. https://doi.org/10.1038/s41585-021-00476-y
Acknowledgements
We thank David Mizrahi for his contribution to the manuscript preparation.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
Conceptualization: Catherine Seet-Lee, Jasmine Yee, and Kate M. Edwards. Formal analysis: Catherine Seet-Lee, Jasmine Yee, Heidi Morahan, Lois S. Ross, and Kate M. Edwards. Writing—review and editing: Catherine Seet-Lee, Jasmine Yee, Heidi Morahan, Lois S. Ross, and Kate M. Edwards.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was not required for this review.
Consent to participate
Consent to participate was not required for this review.
Consent to publish
Consent to publish was not required for this review.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1329 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seet-Lee, C., Yee, J., Morahan, H. et al. The effect of aerobic exercise on tumour blood delivery: a systematic review and meta-analysis. Support Care Cancer 30, 8637–8653 (2022). https://doi.org/10.1007/s00520-022-07132-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07132-0