Abstract
Purpose
Patients receiving cancer treatments experience many treatment-related symptoms. Telehealth is increasingly being used to support symptom management. The overall aim was to determine the effectiveness of nurse-led telehealth symptom management interventions for patients with cancer receiving systemic or radiation therapy compared to usual care on health service use, quality of life, and symptom severity.
Methods
A systematic review was conducted following the Cochrane Handbook and PRISMA reporting guidelines. Five electronic databases were searched. Two independent reviewers screened articles and extracted data. Meta-analysis was performed if data were clinically and methodologically homogeneous. Subanalysis was conducted on reactive and scheduled telehealth interventions.
Results
Of 7749 citations screened, 10 studies were included (8 randomized control trials, 2 quasi-experimental). Five were reactive telehealth interventions with patient-initiated contact and five evaluated scheduled telehealth interventions initiated by nurses. Compared to usual care (typically patient-initiated calls), nurse-led telehealth interventions for symptom management showed no statistically significant difference in hospitalizations, emergency department visits, or unscheduled clinic visits. Two of three studies of reactive telehealth interventions showed improved quality of life. All telehealth interventions showed reduction in the severity of most symptoms. Pain severity was significantly reduced (standard mean difference − 0.54; 95% CI − 0.88, − 0.19). Significant heterogeneity prevented meta-analysis for most outcomes.
Conclusion
Few studies evaluated nurse-led telehealth interventions for cancer symptom management. Compared to usual care, patients exposed to telehealth interventions had reduced symptom severity and no difference in health services use. Future research should focus on better reporting intervention characteristics and consistently measuring outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While undergoing cancer treatment, patients often experience treatment-related symptoms such as fatigue, pain, nausea, constipation, or anxiety [1, 2]. Symptoms are typically monitored during regularly scheduled clinic visits or patients reach out to their clinical team if they have concerns between visits. Given symptoms can affect patients’ daily function and can escalate quickly to be life-threatening, patients often have unscheduled clinic visits, emergency department (ED) visits, hospitalizations, and reduced quality of life (QOL) [3,4,5,6,7]. Hence, symptom management is an essential part of patients’ care throughout treatment and requires collaboration between healthcare professionals and patients [8].
Remote symptom management is provided through telehealth services, where patients can connect at a distance with their healthcare providers between clinic visits. Although telehealth has been used for years, the demand has grown exponentially in the wake of the worldwide COVID-19 pandemic with the need to limit potential exposure, especially for those with high-risk conditions such as cancer [9]. It is therefore important to determine if telehealth is an effective approach for care in the cancer population.
Telehealth uses forms of communication or information technologies to provide remote healthcare to patients [10]. A range of technologies such as telephones, mobile phones, internet-based and smartphone applications have been used for patients with cancer [11,12,13,14,15,16,17]. Telehealth interventions can come in two forms, (1) pre-scheduled communications based on the timing of treatments, typically initiated by the healthcare provider (scheduled interventions), or (2) symptom response communications by the healthcare providers in response to a patient-initiated phone call or an automated alert, triggered by a patient’s self-assessment exceeding a pre-defined threshold (reactive interventions). Patient self-reported outcome (PRO) measures which generate the automated alerts have been shown to improve patient-provider communication and patient satisfaction [18,19,20].
Nurses typically provide telehealth services, including responding to automated alerts, assessing symptom severity, guiding patients in self-care, and triaging those with severe symptoms [21]. Nurse-led telehealth interventions for patients with cancer have been reported to improve patient’s self-care, provide more timely access to resources for symptom management and healthcare professionals, and increase convenience and flexibility [22,23,24]. Despite this, little is known about the effect of telehealth interventions on health service utilization (HSU) and QOL [19, 25,26,27]. Furthermore, previous systematic reviews have focused on specific types of telehealth interventions (telephone or internet-based), have been limited by cancer type, or only included participants being monitored for recurrence once treatment was completed [19, 27,28,29,30].
The overall aim was to determine the effectiveness of nurse-led telehealth symptom management interventions (I) for patients with cancer receiving systemic or radiation therapy (P) compared to usual care (C) on HSU, QOL, and symptom severity (O).
Methods
A systematic review was conducted using the Cochrane Handbook for Systematic Reviews and Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) following an a priori protocol [31, 32].
Eligibility criteria
Based on the PICOS framework, the population of interest (P) was patients with cancer, including all tumor types and stages of disease, receiving systemic (chemotherapy, targeted therapy, immunotherapy, hormonal therapy) or radiation therapy within 4 weeks of study enrollment. There were no participant age restrictions. Interventions (I) included nurse-led telehealth interventions (telephone calls, video technology, emails, web-based systems, text messaging, mobile applications) for cancer symptom monitoring and management [10]. Studies that delivered the intervention by healthcare professionals other than nurses or nurse practitioners (NPs) or included a non-outpatient intervention were excluded. The comparator (C) was usual care. If the control group included another form of telehealth intervention, the study was excluded. The primary outcome (O) was HSU (ED visits, unscheduled hospitalization, unplanned clinic visits). Secondary outcomes included QOL and symptom severity. Included study designs (S) were experimental (quantitative randomized control trials (RCTs), quasi-randomized control trials, non-randomized control trials, and quasi-experimental (controlled before-after (CBA)) and controlled interrupted time series with no date limits. These designs allow for direct comparison of treatment to standard of care while minimizing bias. The study duration was restricted to studies that were at least 4 weeks. Conference abstracts, theses, and dissertations were excluded. Only studies in English were included.
Search strategy and study selection
We searched MEDLINE via OVID, Embase via OVID, Cochrane Central Register of Controlled Trials (CENTRAL); CINAH; PsycINFO; and clinicalTrials.gov (Online Resource 1) as of March 10, 2021, for relevant citations using individualized search strategies for each database. Citations from each database search were exported to Covidence for removing duplicates and screening [33]. Two reviewers (CK, CD, NM) independently screened titles and abstracts for eligibility, and then full-text articles identified for inclusion. At each step, consensus was reached by group discussion to resolve disagreements.
Data extraction
Two reviewers individually extracted data from all included studies, using a standardized data extraction template adapted from the Cochrane handbook [31]. This included study characteristics (study design, aim), participant characteristics (age, gender, cancer type, cancer treatment, inclusion and exclusion criteria), study eligibility, details of telehealth intervention (mode of delivery—telephone, text, web-based application, etc.; healthcare provider respondent—nurse or NP), outcomes measured (HSU, QOL, symptoms), instruments used, results of outcomes including timing of measurement, conflicts of interest, and funding sources. The intervention characteristics were synthesized into a TIDieR table including intervention of interest; intervention (materials and procedures); comparator; provider of intervention; mode of delivery; when and how much; and tailoring [34].
Risk of bias in individual studies
To assess for risk of bias, two reviewers independently appraised the included studies. The Cochrane Collaboration 7-item tool was used to evaluate RCTs (Figs. 1 and 2) [35]. The ROBINS-I tool was used to evaluate non-randomized studies in 7 domains and to assess bias overall [36].
Data analysis
Data was analyzed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions using Review Manager software (RevMan, version 5.4) [31]. HSU was assessed as total number of visits, while post-intervention QOL and symptom scores were extracted as means and standard deviations. The number of patients who provided data for each assessment was extracted, and when ranges were provided, the upper limit was taken to give the most conservative denominator.
Meta-analysis was performed if data was clinically and methodologically homogeneous. Due to different scales used for outcome measures, random-effects models with inverse variance weighting were used to calculate standard mean differences (SMD), with 95% confidence intervals (CIs) in outcomes between intervention and control groups. RCTs and quasi-randomized studies were analyzed separately. Where outcomes were assessed at multiple time points, values from similar post-intervention time points were chosen for analysis. Significant statistical heterogeneity was considered if I2 was greater than 50%, and in such cases, subgroup analyses were performed to assess for possible causes and effects of heterogeneity [31]. Subgroup analysis was performed if data was available, based on the type of telehealth intervention (scheduled vs reactive intervention). Sensitivity analysis was performed to determine the effect of excluding studies with high risk of bias.
When there was insufficient reporting of an outcome or differences in outcome measures (e.g., total days of symptoms rather than symptom scores), such that data could not be combined for meta-analysis, a descriptive synthesis of the outcome was reported. Certainty of findings was evaluated using the GRADE Working Group grades of evidence [37].
Deviations from protocol
There were two deviations from the protocol. Conference abstracts were excluded due to absence of peer review. As a result of limited study numbers, all instruments to measure outcomes were included in this review and none was excluded based on having limited psychometric properties.
Results
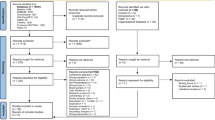
In the search completed on March 21, 2021, there were 7749 citations after duplicates were removed, 178 full-text studies were assessed, and 10 were included (Fig. 3) [11, 14, 17, 23, 38,39,40,41,42,43]. The most common reasons for exclusion during full-text review were trial protocols or ineligible interventions (Online Resource 1). Reviewing the references of the included studies and relevant systematic reviews did not reveal any additional studies.
Study characteristics
Eight RCTs and 2 quasi-experimental studies (CBA design) were included, which evaluated 2315 participants (Table 1). Five studies were conducted in the USA [11, 17, 41,42,43] and one each in Canada [14], France [38], Italy [39], Hong Kong [23], and Turkey [40]. Seven studies evaluated heterogeneous samples of patients with cancer, two studies included patients with lung cancer [40, 43], and one study included women with breast cancer [23]. Nine studies evaluated adults (18 years or older) [11, 17, 23, 38,39,40,41,42,43] and one study evaluated adolescents (12–18 years) [14].
In five studies, nurses or NPs were alerted if self-reported symptoms increased rapidly or were above a pre-defined threshold (reactive intervention) [11, 14, 17, 41, 43]. In the other five studies, nurses or NPs contacted patients at pre-defined times (scheduled interventions) [23, 38,39,40, 42]. Scheduled interventions only used the telephone as a mode of delivery, whereas reactive interventions used telephone-, web-, or smartphone-based systems. Two studies included symptom monitoring in the control arms but the self-reported symptom levels were not provided to the participant’s care team and did not trigger any response, thereby meeting the inclusion criteria [11, 43]. The other control arms addressed symptoms at scheduled clinic visits or by patient-initiated telephone calls. The timing of symptom monitoring and management was highly variable, ranging in frequency from twice daily to monthly (Table 2). Studies also varied in duration from 28 days to 6 months.
Most studies received funding from hospitals, universities, or national-level granting agencies [11, 14, 17, 23, 38, 40,41,42,43]. One study reported industry funding [38], and another did not report funding [39].
Quality of the evidence
Risk of bias assessments of RCTs can be found in Figs. 1 and 2. Due to the nature of the telehealth intervention, nearly all studies were high risk due to limitations in blinding participants. Many studies did not clearly state if HSU information was collected from administrative databases or provided by participants [38, 39, 43]. Basch et al. collected admissions data through medical records, but did not provide clear results on this outcome [17]. Kornblith et al. had a high number of participant dropouts prior to baseline assessment and throughout the study leading to high risk of bias for outcome reporting [41].
Both CBA studies were rated as critical risk of bias as neither commented on attempt to control for possible confounders [14, 40] (Table 3). Outcome measurements were judged to be at serious risk of bias as patients and assessors likely knew if they were in the before or after portion of the study.
Effects of interventions—health services utilization
Four RCTs reported HSU as secondary outcomes [17, 38, 39, 43]. Three of these studies reported ED visits [17, 39, 43], three reported hospitalizations [17, 38, 43], and two reported unscheduled clinic visits [39, 43]. Two studies had reactive interventions [17, 43], and two had scheduled interventions [38, 39]. One study reported HSU as combined outcome (clinic and ED visits) [39]. Yount et al. were the only study to report total number of ED visits, hospital admission, and unscheduled clinic visits separately [43]. A meta-analysis was not able to be performed due to significant heterogeneity in reported outcome measures including composite outcomes and differences in how outcomes were measured.
Compared to usual care, no studies reported a significant improvement in the telehealth intervention groups. Basch et al. found no significant difference between the cumulative incidence of patients who visited the ED (p = 0.16) or were hospitalized (p = 0.75) over a 1-year period for those exposed to reactive telehealth [17]. Over the 12-week study period, Yount et al. reported no statistically significant difference in HSU (ED visits: p = 0.848, hospitalizations: p = 0.877, unscheduled clinic visits: p = 0.129) for those exposed to reactive telehealth [43]. Bouleftour et al. reported the proportion of hospitalized patients who received scheduled telehealth appeared lower at 6, 12, and 24 weeks; these differences were not significant, including at 12 weeks when the difference was most pronounced (p = 0.077) [38]. Cirillo et al. did not find any significant difference in the overall number of patients with at least one ED or unscheduled oncology clinic visit, or visits judged to be unavoidable, after two cycles of chemotherapy for those exposed to scheduled telehealth [39].
Effects of interventions—quality of life
Seven studies assessed QOL [14, 17, 23, 38, 40, 41, 43]. Two studies used the EuroQol EQ-5D [17, 38], two used the Functional Assessment of Cancer Therapy-General (FACT-G) [23, 43], and one each used the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) [41], Functional Living Index-Cancer (FLIC) [40], and the Pediatric Quality of Life Inventory 4.0 (PedsQL 4.0) [14]. Four studies assessed reactive telehealth [14, 17, 41, 43] and three evaluated scheduled telehealth [23, 38, 40].
For the RCTs, there was no significant difference between the intervention and control groups for improving QOL (SMD 0.10; 95% CI − 0.13, 0.34; 4 studies) (Fig. 4: Analysis 1.1) [17, 23, 41, 43]. CBA trials showed no significant difference in post-intervention QOL scores (SMD − 0.02; 95% CI − 0.79, 0.75; 2 studies) [14, 40]. There was significant clinical and statistical heterogeneity (I2 = 63% for RCTs, 80% for CBA studies). The certainty of findings is very low according to GRADE.
Subgroup analysis by intervention type (reactive and scheduled) did not show a significant change in QOL post-intervention scores in RCTs (SMD 0.15; 95% CI − 0.12, 0.42; 3 reactive studies) (Fig. 4: Analysis 1.2) [17, 41, 43]. In a sensitivity analysis, the effect estimate was similar when Kornblith et al. [41] were removed due to high risk of bias for incomplete outcome data. There remained no statistical difference between intervention and control groups.
Effects of interventions—symptom severity
Nine studies (7 RCTs, 2 CBA) reported severity of symptoms as an outcome [11, 14, 23, 38,39,40,41,42,43]. Two studies used the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI CTCAE v4.0) [38, 39], and other studies used the following: Hospital Anxiety and Depression Scale (HADS) [41]; Chemotherapy Symptom Assessment Scale (CSAS) [23]; a numeric severity scale [11]; Memorial Symptom Assessment Scale-Short Form (MSAS-SF) [42]; Patient Health Questionnaire-4 (PHQ-4) [42]; FACT-Lung Symptom Index (FLSI) [43]; Edmonton Symptom Assessment Scale (ESAS) [40]; Brief Pain Inventory (BPI) [14].
Four studies (2 RCTs and 2 CBA) reported post-intervention individual symptom severity scores [14, 23, 40, 41]. Pain was the only symptom with enough data to perform a meta-analysis and was significantly reduced by the telehealth interventions (SMD − 0.54; 95% CI − 0.88, − 0.19) in two CBA studies [14, 40] (Fig. 4: Analysis 1.3). There was no statistically significant heterogeneity (I2 = 0); however, clinically these studies had small sample sizes (40 [14] and 80 [40] participants), assessed very different populations (adolescents [14] vs adults [40]), and had very different interventions (twice daily assessment with a smartphone application (reactive intervention) [14] vs phone calls within the first week after each chemotherapy session (scheduled intervention) [40]). The certainty of findings is very low according to GRADE.
Four studies that evaluated differences between groups found significant symptom reduction (reactive [11, 41], scheduled [23, 40]). In a multicenter trial of patients beginning chemotherapy, Mooney et al. found that intervention participants who self-reported symptoms daily through a telephone voice reporting system and received a reactive call by the NP if symptoms exceeded pre-set thresholds had less symptom severity across all symptoms and lower mean symptom scores for fatigue, pain, sleep problems, nausea/vomiting, depression, anxiety, neuropathy, and sore mouth (p < 0.05) throughout the trial compared to controls [11]. Kornblith et al. reported significantly less anxiety (p < 0.0001) and depression (p < 0.0001) in older patients who received reactive telephone calls if their monthly reported symptom scores exceeded a threshold while receiving cancer treatments in addition to written educational materials, compared to those who just received educational materials [41]. Lai et al. found a significant reduction in 4 of 22 symptoms (mouth/throat problems, fatigue, neuropathy, distressful feelings) assessed during their trial of breast cancer patients receiving adjuvant chemotherapy who received pre-chemotherapy nurse consultation and scheduled nurse telephone calls (within 1 week after the first, second, fourth, and sixth cycles) [23]. Hintistan et al. found symptom severity to be significantly reduced across all domains (p < 0.05: pain, fatigue, nausea, anxiety, anorexia, dyspnea, skin and nail changes, mouth sores, neuropathy) in the intervention group who received scheduled nurse telephone calls within the first week after each chemotherapy session (6 calls), compared to the control group [40].
Asthenia and pain were the most common symptoms in 92 patients who received 15 scheduled nurse telephone calls; however, Bouleftour et al. did not report on severity [38]. In a study of patients receiving oral anticancer treatments, Cirillo et al. did not report any significant difference in the number of participants with symptoms of severe toxicity who received scheduled nurse telephone calls (days 7 and 14 of cycle 1, and day 14 of cycle 2 of chemotherapy) [39]. Traeger et al. found no significant differences in the number of participants in each group having elevated depression or anxiety symptoms at mid- or post-intervention assessments for patients with lung, colorectal, or breast cancer receiving two scheduled telephone calls from an oncology NP during the first week of each of their first two chemotherapy cycles [42].
Discussion
Our systematic review focused on nurse-led telehealth interventions for symptom management in patients with cancer receiving systemic or radiation therapy. Eight RCTs and two quasi-experimental studies met eligibility criteria, assessing 2315 participants [11, 14, 17, 23, 38,39,40,41,42,43]. There were an equal number of studies assessing reactive and scheduled interventions. Individual studies found telehealth interventions were associated with improved QOL and reduction in symptom severity but did not reduce HSU compared to usual care. Meta-analyses, including subgroup analysis, did not reveal any significant difference in QOL scores. Two CBA studies (1 reactive, 1 scheduled) showed significant reduction in pain severity in telehealth intervention groups. Our findings lead to the following points for discussion.
Telephone-based telehealth services were the most common mode of delivery in the included studies. This was not unexpected as it is commonly used by nurses to aid in symptom management [11, 23, 38,39,40,41,42,43,44]. Despite the increase in diversity of telehealth modalities, only two studies evaluated the use of web-based services [14, 17] for symptom monitoring.
Given rising healthcare costs, determining which interventions can lower unnecessary HSU is critical. Despite this, there is limited evidence on how the use of telehealth will impact healthcare costs [29, 45]. The four included studies that measured HSU showed no significant differences between telehealth interventions and usual care [17, 38, 39, 43]. However, there was significant heterogeneity in the included studies with respect to the patient populations, type of intervention, frequency of delivery, and outcomes measured which prevented meta-analysis. These findings are consistent with previous reports that tele-oncology provided no additional HSU benefits compared to in-person services [46].
Many previous reviews have also found methodological limitations and heterogeneity issues when attempting to determine if telehealth would be a beneficial intervention to incorporate into practice [27, 29, 30, 47]. For exclusively internet-based interventions, Moradian et al. concluded that the methodological limitations hindered analysis and called for improved quality research [30]. The lack of significant findings may also be impacted by our measures of HSU which did not include hospital length of stay or direct healthcare costs, both of which could be influenced by telehealth interventions. This is particularly relevant as most studies in our review reported reduced symptom severity for telehealth compared to usual care [11, 14, 23, 40, 41], which may result in shorter hospital admissions and reduced direct healthcare costs. Future studies should consider a more inclusive list of costs when implementing telehealth interventions, particularly in comparison to the costs of providing the telehealth intervention itself.
Telehealth is previously known to reduce the time to access healthcare services, and intervening at the earliest time may provide the best opportunity to see improved QOL [48,49,50,51,52]. Swift intervention is more consistent with the reactive mode of telehealth, as it regularly captures self-reported symptom severity from patients, triggering an alert to their healthcare team who can help with symptom management. In contrast, pre-scheduled symptom monitoring may allow for more frequent contact with patients, but patients may experience a worsening of symptoms between contacts, which could lead to less timely access to care. Despite this, subgroup analysis did not reveal a significant difference in the QOL in either reactive or scheduled intervention studies. This finding may be due to small number of included studies and future research into most effective timing of intervention may be beneficial.
Previous research has shown that patients with complexities, due to chronic conditions, may have improved QOL when using web-based interventions [30, 53]. Two web-based studies included reported improved QOL [14, 17]. One of these studies focused on metastatic patients [17], which adds to the evidence that regularly monitoring symptom severity through web-based applications may be more beneficial in patients with advanced disease and more severe symptoms.
Our review found both reactive and scheduled telehealth studies, which reported reduced severity of symptoms in the intervention group compared to controls. Our findings on reduced symptom severity were similar to other reviews. A systematic review by Moradian et al. found four of six studies reported a reduction in the severity of symptoms for participants receiving internet-based interventions for managing chemotherapy-related symptoms in patients with cancer [30]. Ream et al.’s systematic review of 32 studies found that telephone-delivered interventions for symptom management in adults with cancer were effective for symptoms such as depression, anxiety, fatigue, and emotional distress [27]. In the systematic review of 20 studies by Chen et al., telehealth interventions improved quality of life, self-efficacy and depression, distress, and perceived stress in patients with breast cancer [47]. Previous reviews did not compare reactive to scheduled interventions, or specifically assess nurse-led interventions. The results of this review are consistent with previous systematic reviews in this area which reported limited availability of studies, methodological limitations, and heterogeneity [27, 29, 30, 47].
This review focused on nurse-led telehealth interventions for symptom management and did not include studies which assessed post-treatment follow-up programs [29], education programs [27, 47], psychological interventions [27, 47], or patient’s carers or partners [27] which were included in other reviews. The implementation of these additional programs/interventions may provide additional benefits but may also be more costly, requiring additional expertise that may not be widely available within ambulatory oncology centers. For example, the degree of training and expertise influences the success of telehealth symptom management programs. Providing training workshops for nurses on the use of evidence-informed practice guides for telephone-based symptom support has been shown to improve nurses’ confidence in providing symptom support and using the practice guides [54]. However in this review, the descriptions of educational preparation of nurses providing telehealth services were variable across studies and it was impossible to determine the influence of their educational preparation on the outcomes of the telehealth interventions.
Strengths and limitations of review
A strength of this review was the high methodological quality. According to AMSTAR II, this review met 15 of 16 items [55]. The missing item was not investigating for publication bias. Although this review allowed for greater variety of telehealth interventions and cancer types not previously included in other reviews, there are some limitations that should be considered when interpreting the findings. There was heterogeneity due to differences in the intervention design, populations, and outcome reporting that interfered with our ability to combine findings across studies. Limiting inclusion to nurse-led telehealth interventions missed studies of telehealth interventions provided by other healthcare professionals. This review focused on HSU as a surrogate for health system costs but did not directly assess costs.
Conclusion
Patients with cancer undergoing systemic and radiation therapies may develop symptoms that result in visits to the ED or hospitalization. This review focused on nurse-led telehealth interventions for symptom management in patients with cancer as a possible method of reducing HSU. Although it did not find any evidence that telehealth interventions reduced HSU or improved QOL, there was some evidence for decreased severity of symptoms from both reactive and scheduled telehealth interventions. Synthesizing findings across studies is limited due to significant heterogeneity in the telehealth interventions, participants, and outcomes measured. Future research can improve the quality of evidence in this area by evaluating interventions more consistent with current practice for patients receiving cancer treatments and providing more consistent reporting of outcomes.
Supplementary information.
Data availability
Not applicable.
Code availability
Not applicable.
References
Macartney G, Stacey D, Carley M, Harrison MB (2012) Priorities, barriers and facilitators for remote support of cancer symptoms: a survey of Canadian oncology nurses. Can Oncol Nurs J 22:235–240. https://doi.org/10.5737/1181912x224235240
Stark L, Tofthagen C, Visovsky C, McMillan SC (2012) The symptom experience of patients with cancer. J Hosp Palliat Nurs 14:61–70. https://doi.org/10.1097/NJH.0b013e318236de5c
Foltran L, Aprile G, Pisa FE, Ermacora P, Pella N, Iaiza E, Poletto E, Lutrino SE, Mazzer M, Giovannoni M, Cardellino GG, Puglisi F, Fasola G (2014) Risk of unplanned visits for colorectal cancer outpatients receiving chemotherapy: a case-crossover study. Support Care Cancer 22:2527–2533. https://doi.org/10.1007/s00520-014-2234-z
Vandyk AD, Harrison MB, Macartney G, Ross-White A, Stacey D (2012) Emergency department visits for symptoms experienced by oncology patients: a systematic review. Support Care Cancer 20:1589–1599. https://doi.org/10.1007/s00520-012-1459-y
Enright K, Grunfeld E, Yun L, Moineddin R, Ghannam M, Dent S, Eisen A, Trudeau M, Kaizer L, Earle C, Krzyzanowska MK (2015) Population-based assessment of emergency room visits and hospitalizations among women receiving adjuvant chemotherapy for early breast cancer. J Oncol Pract 11:126–132. https://doi.org/10.1200/JOP.2014.001073
Arndt V, Stegmaier C, Ziegler H, Brenner H (2006) A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer 107:2496–2503. https://doi.org/10.1002/cncr.22274
Livingston PM, Craike M, Considine J (2011) Unplanned presentations to emergency departments due to chemotherapy induced complications: opportunities for improving service delivery. Australas Emerg Nurs J 14:62–68. https://doi.org/10.1016/j.aenj.2011.03.005
Prip A, Møller KA, Nielsen DL, Jarden M, Olsen M-H, Danielsen AK (2018) The patient-healthcare professional relationship and communication in the oncology outpatient setting: a systematic review. Cancer Nurs 41:E11–E22. https://doi.org/10.1097/NCC.0000000000000533
Koonin LM, Hoots B, Tsang CA, Leroy Z, Farris K, Jolly T, Antall P, McCabe B, Zelis CBR, Tong I, Harris AM (2020) Trends in the use of telehealth during the emergence of the COVID-19 pandemic - United States, January-March 2020. MMWR Morb Mortal Wkly Rep 69:1595–1599. https://doi.org/10.15585/mmwr.mm6943a3
Canadian Agency for Drugs and Technologies in Health (2016) Telehealth: summary of evidence | CADTH. https://cadth.ca/telehealth-summary-evidence. Accessed 5 Oct 2021
Mooney KH, Beck SL, Wong B, Dunson W, Wujcik D, Whisenant M, Donaldson G (2017) Automated home monitoring and management of patient-reported symptoms during chemotherapy: results of the symptom care at home RCT. Cancer Med 6:537–546. https://doi.org/10.1002/cam4.1002
Foster C, Grimmett C, May CM, Ewings S, Myall M, Hulme C, Smith PW, Powers C, Calman L, Armes J, Breckons M, Corner J, Fenlon D, Batehup L, Lennan E, May CR, Morris C, Neylon A, Ream E, Turner L, Yardley L, Richardson A (2016) A web-based intervention (RESTORE) to support self-management of cancer-related fatigue following primary cancer treatment: a multi-centre proof of concept randomised controlled trial. Support Care Cancer 24:2445–2453. https://doi.org/10.1007/s00520-015-3044-7
Galiano-Castillo N, Cantarero-Villanueva I, Fernández-Lao C, Ariza-García A, Díaz-Rodríguez L, Del-Moral-Ávila R, Arroyo-Morales M (2016) Telehealth system: a randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer 122:3166–3174. https://doi.org/10.1002/cncr.30172
Jibb LA, Stevens BJ, Nathan PC, Seto E, Cafazzo JA, Johnston DL, Hum V, Stinson JN (2017) Implementation and preliminary effectiveness of a real-time pain management smartphone app for adolescents with cancer: a multicenter pilot clinical study. Pediatr Blood Cancer 64:.https://doi.org/10.1002/pbc.26554
Mosher CE, Winger JG, Hanna N, Jalal SI, Einhorn LH, Birdas TJ, Ceppa DP, Kesler KA, Schmitt J, Kashy DA, Champion VL (2016) Randomized pilot trial of a telephone symptom management intervention for symptomatic lung cancer patients and their family caregivers. J Pain Symptom Manage 52:469–482. https://doi.org/10.1016/j.jpainsymman.2016.04.006
Weaver A, Young A, Rowntree J, Townsend N, Pearson S, Smith J, Gibson O, Cobern W, Larsen M, Tarassenko L (2007) Application of mobile phone technology for managing chemotherapy-associated side-effects. Ann Oncol 18:1887–1892. https://doi.org/10.1093/annonc/mdm354
Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, Rogak L, Bennett AV, Dueck AC, Atkinson TM, Chou JF, Dulko D, Sit L, Barz A, Novotny P, Fruscione M, Sloan JA, Schrag D (2016) Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 34:557–565. https://doi.org/10.1200/JCO.2015.63.0830
Basch E, Artz D, Dulko D, Scher K, Sabbatini P, Hensley M, Mitra N, Speakman J, McCabe M, Schrag D (2005) Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol 23:3552–3561. https://doi.org/10.1200/JCO.2005.04.275
Chen J, Ou L, Hollis SJ (2013) A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res 13:211. https://doi.org/10.1186/1472-6963-13-211
Detmar SB, Muller MJ, Schornagel JH, Wever LDV, Aaronson NK (2002) Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA 288:3027–3034. https://doi.org/10.1001/jama.288.23.3027
Stacey D, Carley M, Kohli J, Skrutkowski M, Avery J, Bazile AM, Court A, Nagel DA, Budz D (2014) Remote symptom support training programs for oncology nurses in Canada: an environmental scan. Can Oncol Nurs J 24:78–88. https://doi.org/10.5737/1181912x2427882
Moss EL (2014) “Just a telephone call away”: transforming the nursing profession with telecare and telephone nursing triage. Nurs Forum 49:233–239. https://doi.org/10.1111/nuf.12052
Lai XB, Ching SSY, Wong FKY, Leung CWY, Lee LH, Wong JSY, Lo YF (2019) A nurse-led care program for breast cancer patients in a chemotherapy day center: a randomized controlled trial. Cancer Nurs 42:20–34. https://doi.org/10.1097/NCC.0000000000000539
Stacey D, Green E, Ballantyne B, Skrutkowski M, Whynot A, Tardif L, Tarasuk J, Carley M, Pan-Canadian Oncology Symptom Triage and Remote Support (COSTaRS) Team (2016) Patient and family experiences with accessing telephone cancer treatment symptom support: a descriptive study. Supportive Care Cancer 24:893–901. https://doi.org/10.1007/s00520-015-2859-6
Gustavell T, Sundberg K, Segersvärd R, Wengström Y, Langius-Eklöf A (2019) Decreased symptom burden following surgery due to support from an interactive app for symptom management for patients with pancreatic and periampullary cancer. Acta Oncol 58:1307–1314. https://doi.org/10.1080/0284186X.2019.1633473
Kroenke K, Theobald D, Wu J, Norton K, Morrison G, Carpenter J, Tu W (2010) Effect of telecare management on pain and depression in patients with cancer: a randomized trial. JAMA 304:163–171. https://doi.org/10.1001/jama.2010.944
Ream E, Hughes AE, Cox A, Skarparis K, Richardson A, Pedersen VH, Wiseman T, Forbes A, Bryant A (2020) Telephone interventions for symptom management in adults with cancer. Cochrane Database Syst Rev 6:CD007568. https://doi.org/10.1002/14651858.CD007568.pub2
Agboola SO, Ju W, Elfiky A, Kvedar JC, Jethwani K (2015) The effect of technology-based interventions on pain, depression, and quality of life in patients with cancer: a systematic review of randomized controlled trials. J Med Internet Res 17:e65. https://doi.org/10.2196/jmir.4009
Dickinson R, Hall S, Sinclair JE, Bond C, Murchie P (2014) Using technology to deliver cancer follow-up: a systematic review. BMC Cancer 14:311. https://doi.org/10.1186/1471-2407-14-311
Moradian S, Voelker N, Brown C, Liu G, Howell D (2018) Effectiveness of Internet-based interventions in managing chemotherapy-related symptoms in patients with cancer: a systematic literature review. Support Care Cancer 26:361–374. https://doi.org/10.1007/s00520-017-3900-8
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V (2021) Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 350:g7647. https://doi.org/10.1136/bmj.g7647
Covidence systematic review software. Veritas Health Innovation, Melbourne, Australia
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan A-W, Michie S (2014) Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 348:g1687. https://doi.org/10.1136/bmj.g1687
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan A-W, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. https://doi.org/10.1136/bmj.i4919
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926. https://doi.org/10.1136/bmj.39489.470347.AD
Bouleftour W, Muron T, Guillot A, Tinquaut F, Rivoirard R, Jacquin J-P, Saban-Roche L, Boussoualim K, Tavernier E, Augeul-Meunier K, Collard O, Mery B, Pupier S, Oriol M, Bourmaud A, Fournel P, Vassal C (2021) Effectiveness of a nurse-led telephone follow-up in the therapeutic management of patients receiving oral antineoplastic agents: a randomized, multicenter controlled trial (ETICCO study). Supportive Care Cancer. https://doi.org/10.1007/s00520-020-05955-3
Cirillo M, Carlucci L, Legramandi L, Baldini E, Sacco C, Zagonel V, Leo S, Di Fabio F, Tonini G, Meacci ML, Tartarone A, Farci D, Tortora G, Zaninelli M, Valori VM, Cinieri S, Carrozza F, Barbato E, Fabbroni V, Cretella E, Gamucci T, Lunardi G, Zamboni S, Micallo G, Cascinu S, Pinto C, Gori S (2020) Oral anticancer therapy project: clinical utility of a specific home care nursing programme on behalf of Italian Association of Medical Oncology (AIOM). J Clin Nurs 29:119–129. https://doi.org/10.1111/jocn.15064
Hintistan S, Nural N, Cilingir D, Gursoy A (2017) Therapeutic effects of nurse telephone follow-up for lung cancer patients in Turkey. Cancer Nurs 40:508–516. https://doi.org/10.1097/NCC.0000000000000461
Kornblith AB, Dowell JM, Herndon JE 2nd, Engelman BJ, Bauer-Wu S, Small EJ, Morrison VA, Atkins J, Cohen HJ, Holland JC (2006) Telephone monitoring of distress in patients aged 65 years or older with advanced stage cancer: a cancer and leukemia group B study. Cancer 107:2706–2714. https://doi.org/10.1002/cncr.22296
Traeger L, McDonnell TM, McCarty CE, Greer JA, El-Jawahri A, Temel JS (2015) Nursing intervention to enhance outpatient chemotherapy symptom management: patient-reported outcomes of a randomized controlled trial. Cancer 121:3905–3913. https://doi.org/10.1002/cncr.29585
Yount SE, Rothrock N, Bass M, Beaumont JL, Pach D, Lad T, Patel J, Corona M, Weiland R, Del Ciello K, Cella D (2014) A randomized trial of weekly symptom telemonitoring in advanced lung cancer. J Pain Symptom Manage 47:973–989. https://doi.org/10.1016/j.jpainsymman.2013.07.013
Oncology Nursing Program (2019) Oncology nursing telepractice standards. Cancer Care Ontario
Beaver K, Hollingworth W, McDonald R, Dunn G, Tysver-Robinson D, Thomson L, Hindley AC, Susnerwala SS, Luker K (2009) Economic evaluation of a randomized clinical trial of hospital versus telephone follow-up after treatment for breast cancer. Br J Surg 96:1406–1415. https://doi.org/10.1002/bjs.6753
Sirintrapun SJ, Lopez AM (2018) Telemedicine in cancer care. Am Soc Clin Oncol Educ Book 38:540–545. https://doi.org/10.1200/EDBK_200141
Chen Y-Y, Guan B-S, Li Z-K, Li X-Y (2018) Effect of telehealth intervention on breast cancer patients’ quality of life and psychological outcomes: a meta-analysis. J Telemed Telecare 24:157–167. https://doi.org/10.1177/1357633X16686777
Forkner-Dunn J (2003) Internet-based patient self-care: the next generation of health care delivery. J Med Internet Res 5:e8. https://doi.org/10.2196/jmir.5.2.e8
Bhavnani SP, Narula J, Sengupta PP (2016) Mobile technology and the digitization of healthcare. Eur Heart J 37:1428–1438. https://doi.org/10.1093/eurheartj/ehv770
Marcolino MS, Oliveira JAQ, D’Agostino M, Ribeiro AL, Alkmim MBM, Novillo-Ortiz D (2018) The impact of mHealth interventions: systematic review of systematic reviews. JMIR Mhealth Uhealth 6:e23. https://doi.org/10.2196/mhealth.8873
Kim BY, Lee J (2017) Smart devices for older adults managing chronic disease: a scoping review. JMIR Mhealth Uhealth 5:e69. https://doi.org/10.2196/mhealth.7141
Cleeland CS, Wang XS, Shi Q, Mendoza TR, Wright SL, Berry MD, Malveaux D, Shah PK, Gning I, Hofstetter WL, Putnam JB, Vaporciyan AA (2011) Automated symptom alerts reduce postoperative symptom severity after cancer surgery: a randomized controlled clinical trial. J Clin Oncol 29:994–1000. https://doi.org/10.1200/JCO.2010.29.8315
Yun YH, Lee KS, Kim Y-W, Park SY, Lee ES, Noh D-Y, Kim S, Oh JH, Jung SY, Chung K-W, Lee YJ, Jeong S-Y, Park KJ, Shim YM, Zo JI, Park JW, Kim YA, Shon EJ, Park S (2012) Web-based tailored education program for disease-free cancer survivors with cancer-related fatigue: a randomized controlled trial. J Clin Oncol 30:1296–1303. https://doi.org/10.1200/JCO.2011.37.2979
Stacey D, Skrutkowski M, Carley M, Kolari E, Shaw T, Ballantyne B (2015) Training oncology nurses to use remote symptom support protocols: a retrospective pre-/post-study. Oncol Nurs Forum 42:174–182. https://doi.org/10.1188/15.ONF.174-182
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358:j4008. https://doi.org/10.1136/bmj.j4008
Acknowledgements
We wish to acknowledge Dr. Dean Fergusson, Dr. Matthew McInnes, and Sasha Mazzarello for their methodological guidance throughout the study. We would like to also thank Lindsey Sikora and Marie-Cécile Domecq at the University of Ottawa Library for their assistance in the design of the search strategy.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Search strategy and analysis was performed by CK. Study screening, data extraction, and preparing the first draft of the manuscript were performed by CK, CD, and NM. All the co-authors listed contributed to reviewing and revising the manuscript and have approved it for submission. The protocol was not registered but is available upon request.
Corresponding author
Ethics declarations
Ethics approval
This is a systematic review and meta-analysis; ethics approval is not required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chanel Kwok and Charlena Degen are co-lead.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kwok, C., Degen, C., Moradi, N. et al. Nurse-led telehealth interventions for symptom management in patients with cancer receiving systemic or radiation therapy: a systematic review and meta-analysis. Support Care Cancer 30, 7119–7132 (2022). https://doi.org/10.1007/s00520-022-07052-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07052-z