Abstract
Background
Studies in 1983 and 1993 identified and ranked symptoms experienced by cancer patients receiving chemotherapy. We repeated the studies to obtain updated information on patient perceptions of chemotherapy-associated symptoms.
Patients and methods
A cross-sectional interview and patient-reported outcome questionnaires were administered to out-patients receiving chemotherapy. Patients selected from 124 cards to identify and rank the severity of physical and non-physical symptoms they had experienced and attributed to chemotherapy (primary endpoint). The patient’s medical oncologist and primary chemotherapy nurse were invited to rank the five symptoms they believed the patient would rank as their most severe. We analysed the association of symptoms and their severity with patient demographics, chemotherapy regimen, and patient-reported outcomes. Results were compared to the earlier studies.
Results
Overall, 302 patients completed the interview: median age 58 years (range 17–85); 56% female; main tumour types colorectal 81 (27%), breast 67 (22%), lung 49 (16%); 45% treated with curative intent. Most common symptoms (reported by >50%) were: alopecia, general weakness, effects on family/partner, loss of taste, nausea, fatigue, difficulty sleeping, effects on work/home duties, and having to put life on hold. The most severe symptoms (ranked by >15% in top five) were: concern about effects on family/partner, nausea, fear of the future, fatigue, not knowing what will happen, putting my life on hold, and general weakness. Perceptions of doctors and nurses of patients’ symptom severity closely matched patients’ rankings.
Conclusions
Compared to earlier studies, there was an increase in non-physical concerns such as effects on family and future, and a decrease in physical symptoms, particularly vomiting, but nausea, fatigue and general weakness remained bothersome.
Highlights
• Symptoms related to chemotherapy have changed over time, likely due to less toxic regimens and improvements in supportive care.
• Effects on family/partner, fear of the future, not knowing what will happen, and “life on hold” were major issues for patients.
• Vomiting has decreased but nausea, fatigue and general weakness remain common symptoms for chemotherapy patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Two studies published in 1983 [1] and 1993 [2] identified and ranked symptoms experienced by patients with cancer who were receiving chemotherapy. In 1983, 99 patients who were receiving chemotherapy reported that non-physical side effects constituted 54% of the 15 most severe symptoms; these included the thought of coming for treatment, length of time treatment takes, and having to have a needle. Major physical side effects were vomiting, nausea and hair loss. When physical and non-physical categories were combined, vomiting, nausea and hair loss remained the most severe [1].
A decade later, patients reported a reduction in the severity of some symptoms, particularly vomiting, and a shift from concerns about physical to psychosocial issues. Nausea was the most severe symptom followed by tiredness and alopecia. Vomiting was of lesser concern, reflecting the introduction of 5HT3 antagonist anti-emetics. Concern about the effect on friends and family increased in rankings from 10th to 3rd. In both studies, differences were seen in the symptoms experienced and their severity, based on chemotherapy regimen, age, and sex [2].
Similar methodology was applied to 100 French patients with advanced cancer, recruited between 1998 and 2000 [3]. The side-effect identified as most common and severe was “affects my family or partner”, followed by “loss of hair”, then “constantly tired”.
Since 2000, there have been major changes in chemotherapy and in supportive care to manage side-effects and symptoms, increased patient involvement in cancer care decisions, and people wanting more information about their treatment including side-effects [4,5,6]. Here, we provide information on patient perceptions of chemotherapy-associated symptoms in people being treated with modern chemotherapy and supportive care including anti-emetic regimens.
Methods
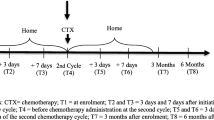
This cross-sectional survey was conducted as a face-to-face interview with patient-reported outcome measures (PROM) on one occasion when patients were attending out-patient medical oncology clinics at two metropolitan teaching hospitals (one of which was included in the prior studies) and one regional hospital, between May 2008 and October 2016. Patients with any stage cancer, who were receiving chemotherapy, were invited to participate in the study. Eligibility criteria included a diagnosis of invasive cancer, currently receiving chemotherapy for a solid malignancy and completion of at least one cycle, and sufficient English to complete PROM.
Procedures
The methodology used in the previous studies [1, 2], with additional questions, was retained to maximise validity of comparisons. Additional symptoms were added to the original list by an expert group that consisted of two medical oncologists, a senior cancer nurse, social worker, clinical psychologist, and behavioural scientist. The additional items (highlighted in Table 1) were piloted on 10 patients to assess understanding and construct validity.
The patient interview was scripted and was conducted by trained research assistants. The procedure was as described in the previous two studies [1, 2]. In brief, two sets of white cards were prepared. Group A listed physical side-effects of chemotherapy (n = 69) and Group B listed non-physical side-effects of chemotherapy (n = 55) (Table 1). Both card sets were shuffled, and Group A cards were presented first, one at a time. The participant was asked to select all cards that described a symptom they had experienced and that they attributed to their current chemotherapy. They were then asked to rank the selected cards in order of severity. This process was repeated for Group B cards. The five highest ranked cards from each group were combined and the patient was asked to select and rank the five most severe symptoms regardless of group. Five points were allocated to symptoms ranked as the most severe, decreasing to one point for symptoms ranked as least severe. The allocated points were used to generate an overall ranking of symptom severity. The patient’s medical oncologist and a nurse closely involved in their care were also asked to rank the five symptoms they believed that each patient would rank as their most severe. They were given a separate list of physical and non-physical symptoms, grouped by systems or domains, and asked firstly to rank the top 5 for each list. They were then asked to rank the top 5 across both symptom lists.
Demographic, disease, and treatment characteristics were collected from the participant and their medical record. The research assistant rated the participant’s performance status based on European Co-operative Oncology Group (ECOG) criteria [7], and determined their Colinet Simplified Comorbidity Score [8].
An additional component done after the patient interview was completion of several health-related quality of life PROMs. These included self-rating of ECOG performance status [7], quality of life (QOL) and fatigue assessed by the Functional Assessment of Cancer Therapy – General (FACT-G) and Fatigue (FACT-F) subscale [9, 10], anxiety and depression assessed by the 12-item General Health Questionnaire (GHQ-12) [11], and the EQ-5D Thermometer Scale of overall health state [12]. In addition, patients retrospectively completed linear analogue self-assessment (LASA) scales documenting the severity of anticipatory nausea and vomiting, and nausea and vomiting within and more than 24 h after chemotherapy for their last cycle of chemotherapy.
Ethical approval was granted by each hospital and all participants provided written, informed consent.
Statistical analysis
The 1983 and 1993 studies had 99 and 150 patients respectively. We increased the planned sample size to 400 patients to increase the generalisability of the study and to determine if there was a difference across the number of chemotherapy cycles. Statistical analyses were conducted using Stata 13.
The primary aim was to determine the most severe symptoms experienced by cancer patients while receiving chemotherapy. Descriptive statistics were used to describe the frequency of symptoms and to rank their severity. Major secondary goals included: (i) comparison of symptoms with those reported in the studies conducted in 1983 and 1993; (ii) the association of symptoms with patient characteristics such as sex, age, chemotherapy regimen (platinum-based, taxane, and other), number of prior chemotherapy cycles and PROMs.; (iii) comparison of patients’ ratings of symptom severity with that of their physicians and nurses.
For the LASA scales, scores ranged from 0 (no discomfort) to 100 (extreme discomfort). Consistent with the 1993 study, a score of >75 was interpreted as indicating a substantial level of discomfort. Proportions were reported.
Logistic regression models were used to analyse the association of symptoms and their severity with patient demographics, chemotherapy regimen and PROMs, except for PROMs measured on a continuous scale when linear regression models were used. The regression models used the demographic, treatment or PROM variable as the dependent variable and each of the symptoms as exploratory/independent variables. Consistent with the previous studies, we restricted the analysis to comparing whether each symptom was included among the five most severe.
The 1983 study included only patients undergoing treatment for advanced cancer, whereas the 1993 study included people with earlier stage disease, so for comparison between studies we restricted analysis only to those with advanced/metastatic cancer. Raw data were not available from the previous studies, so we compared severity rankings for symptoms.
Results
The study was completed over two time periods from 2008 to 2013 (n = 272 patients) and from January to October 2016 (n = 30) due to limitations in resources.
Of 391 patients approached, 308 consented to participate and 302 completed the assessment (Fig. 1, Consort diagram). Reasons for declining to participate were: felt too unwell; lack of interest; insufficient English; and unable to schedule.
The median age of participants was 58 years (range 17–85) and 56% were female (Table 2). The predominant tumour types were colorectal (27%), breast (22%), and lung (16%). Forty-five percent were being treated with curative intent. The median time since diagnosis was 6 months (range 1–392). In total, 54% of patients were receiving a platinum-based chemotherapy regimen, and 31% a taxane-containing regimen. The median number of cycles received prior to the interview was 4 (range 1–23).
Symptoms experienced and severity
Patients reported experiencing a median of 29 symptoms: 18 physical and 11 non-physical. The most common symptoms were loss of hair, general weakness, concern about effects on family or partner, loss of taste, nausea, fatigue, difficulty sleeping, concern about effects on work or home duties, and having to put life on hold. These were reported by >50% of patients. (Table 3, Supplementary Tables 1 and 2). While other symptoms were less commonly reported, 58 of 124 symptoms were reported by >25% of patients.
Association of symptoms with patient characteristics
Differences associated with the chemotherapy regimen, tumour type and with number of cycles were most often physical symptoms (Supplementary Tables 3 and 4). Patients on platinum-based regimens were more likely to report cough and pain when swallowing, with pins and needles, general weakness, loss of appetite and indigestion rated as more severe compared with other regimens (Supplementary Table 3 and 4). Patients receiving taxanes were more likely to report loss of hair (and to rate it as more severe), general aches and pains, and skin rash. There were no significant differences across drug regimens in the non-physical symptoms regarded as most severe.
Patients who had received more cycles of chemotherapy were more likely to report pins and needles, diarrhoea, abdominal pain, and thrush, with headaches and sore/tender muscles being rated more severe. They were also more likely to report feeling their cancer made them different. In contrast, they were less likely to report symptoms like indigestion, joint aches and pains, and ringing ears.
Women were more likely than men to report many physical and non-physical symptoms including loss of hair, pins and needles, headache, slow thinking, crying more often, and feeling unattractive. The only symptoms reported more frequently by men were passing more urine and hiccups, with trouble swallowing rated as more severe. Younger patients (under 60 years) were more likely to report concerns about the effects on work or home duties as well as difficulty sleeping and feeling angry. Older patients were more likely to report shortness of breath and easy bruising, with general weakness, changes in taste and constipation rated as more severe.
Patients treated with curative intent were more likely than those with advanced disease to report physical symptoms such as hot flushes, changes in smell, and sore eyes as well as non-physical symptoms like crying more often, seeing very sick people, and feeling of having to have treatment I don’t want (Supplementary Table 3). They were also more likely to rate as severe alopecia and feeling treatment is damaging (Supplementary Table 4). Patients treated with palliative intent were more likely to report loss of appetite, abdominal pain, cough, and feeling that cancer makes me different, no end to treatment and not understanding what is happening.
The most severe symptom was concern about the effects on family or partner, with 34% of patients ranking it among their five most severe symptoms and 12% as the most severe (Table 3). The next most severe symptoms were nausea, fear of the future, fatigue, not knowing what will happen, having to put my life on hold, and general weakness; these were ranked by >15% of patients among their five most severe symptoms.
Severity of several non-physical symptoms, including having to put life on hold and cost of treatment, were associated with lower overall quality of life as measured by FACT-G and higher levels of depression and anxiety as measured by GHQ-12 (Table 4). Having to put life on hold and severe fatigue were associated with worse self-reported ECOG performance status. Other PROMS reflected well the patient reported symptoms.
Comparison of patients’ symptom severity with that of doctors and nurses
Symptoms ratings by doctors and nurses were available for 96 and 116 patients respectively: their ranking of symptom severity was similar to the full sample. Perceptions of doctors and nurses matched closely the patients’ own rankings (Table 3). Six of the 10 symptoms ranked most severe by patients were also ranked by doctors and nurses among the 10 most severe. Having to put life on hold, difficulty sleeping, and constipation were ranked more highly by patients than doctors or nurses. Length of time spent at clinic and in waiting for chemotherapy, vomiting, and having to have a needle were ranked as more severe by doctors and nurses than by patients.
Changes in symptom severity since 1983 and 1993 in people with advanced cancer
Vomiting and nausea declined in the severity ranking across study years. Vomiting, ranked as the most severe symptom in 1983 and fifth most severe in 1993, was ranked 23rd among symptoms of patients with advanced disease in the current study (Supplementary Table 5). Nausea, ranked as the most severe symptom in 1993, was ranked as the fifth in the current study. Conversely, concerns about effects on family increased in severity (ranked 10th in 1983, fourth in 1993 and first in the current study). Depression decreased in severity, falling to 51st in the current study while the ranking of anxiety remained more stable. Some of the non-physical symptoms that ranked highly in terms of severity in the current study, such as fear of the future, and feeling like my life is on hold, were not surveyed in the previous studies.
Nausea and vomiting pre, within and post 24-h of chemotherapy
Only 1% of patients in the current study had substantial anticipatory nausea (score of >75), and no patient reported substantial anticipatory vomiting compared with 17% and 5% in the previous studies (Table 5). Within 24 h of chemotherapy, 8% of patients reported substantial nausea and 4% substantial vomiting compared with 51% and 24% previously. Beyond 24 h after chemotherapy, 10% reported severe nausea and 4% severe vomiting compared with 57% and 29% in the previous studies. The results from LASA scales are consistent with the symptom ranking and indicate that the severity of nausea and vomiting has declined substantially since 1983.
Vomiting during the 24 h after chemotherapy and delayed vomiting after 24 h were strongly associated with higher ranking of vomiting whereas anticipatory vomiting was not. In contrast, anticipatory nausea, in addition to nausea within 24 h and after 24 h of chemotherapy, was a strong predictor of patients’ nausea ranking.
Discussion
It is almost 40 years since the original ‘On the Receiving End’ study was published. In 1983, the top five severe symptoms were: vomiting, nausea, loss of hair, thought of coming for treatment, and length of time treatment takes, which was especially a concern for men. With improvement in management of acute physical symptoms, particularly vomiting, there has been a shift to patients reporting greater concern with non-physical side effects, particularly the impact of their cancer and/or treatment on their partner and family. In the current study, this was rated in the top five most severe symptoms by 34% and as the most severe by 12% of participants. By comparison, it was tenth in severity in 1983. Other highly rated symptoms in our study included fear of the future, uncertainty of what will happen and having to put their life on hold, which were not options in the earlier studies.
While major advances have been made in the prevention and treatment of vomiting, nausea remains difficult to manage and debilitating for patients [13]. Fatigue also continues to be a major problem with 56% of patients reporting fatigue and 66% general weakness. Our longitudinal study in colorectal cancer patients found that 70% of patients reported fatigue immediately following adjuvant chemotherapy compared with 31% who had surgery alone and 22% of healthy controls [14], with 44% still reporting fatigue 6 months after chemotherapy compared to ~30% of non-chemotherapy patients and controls. Studies in women with breast cancer have reported similar results [15, 16].
In the present study, 45% of patients were being treated with curative intent, most with a platinum or taxane regimen, and all patients were treated in the outpatient department. In the original 1983 study, all participants had advanced cancer and many required treatment administration as inpatients. Differences were more likely to be in physical symptoms across chemotherapy regimens rather than non-physical symptoms. By comparison, the 1993 study included a third of patients being treated with adjuvant chemotherapy.
Patients are generally better prepared for physical symptoms with chemotherapy than they are for psychosocial issues. This is likely because many health care professionals focus more on physical side-effects when providing information and obtaining informed consent prior to chemotherapy [17] and in eliciting symptoms in subsequent consultations [18, 19]. With improved physical outcomes, fear of cancer recurrence or progression, and psychosocial issues have become more salient. The similarities in non-physical symptoms ranked as severe across treatment regimens suggests the commonality of these concerns across tumour types and a need to address them more effectively. Results of the present study, and other studies showing cancer survivors have high psychological distress [20], fear of cancer recurrence, increased use of health services and poorer quality of life [21], suggest that mental health and well-being in cancer patients require greater attention.
Detecting and monitoring of symptoms experienced by patients receiving chemotherapy is an essential component of quality care. Changes in symptom profile may necessitate modification to the treatment regimen, or the provision of additional supportive care and patient education. Several studies have shown that clinicians tend to underestimate the incidence and severity of symptoms when compared to patient self-report [22,23,24]. Clinician accuracy in detecting patient symptoms has been reported to be lower for more subjective symptoms (e.g. fatigue and dyspnoea) than for symptoms that can be observed directly (e.g. vomiting and diarrhoea) [24]. However, in the present study, the perceptions of the oncologists and cancer nurses in rating the top five symptoms were fairly consistent with patient self-report, with oncologist awareness of troublesome non-physical symptoms greater than expected.
Limitations and strengths
Our study provides important updated information to the oncology community regarding the symptoms that patients undergoing chemotherapy experience. We acknowledge that this may be different to the symptoms they are most concerned about, and that financial concerns are likely to be much greater in countries that do not have universal health care. We did not meet our planned sample size of 400, but recruited ~300 participants covering a broad spectrum of tumour type, chemotherapy regimens and disease stage. All patients were currently receiving chemotherapy and we investigated a wide array of symptoms. Data collection was spread over several years and there may have been changes in treatment and supportive care during this time which impacted symptoms. Targeted therapies and immunotherapy are not captured in this study with the exception of 6% of participants receiving a targeted therapy (e.g., trastuzumab) together with chemotherapy.
We recognise that there is confounding between some variables (for example all breast cancer patients were female, and some chemotherapy regimens are used only for certain cancer types), which limits the analysis of symptoms as a function of type of cancer, age and sex. Our matched data between patients and staff was limited to ~100 patients. Although the ranking of symptom severity by patients was similar to the larger cohort, it is possible that there was self-selection of oncologists and nurses who were more likely to discuss concerns with their patients. If so, we may have over-estimated their appreciation of symptoms most important to patients, but we would like to think that this may be due to oncology staff increasingly engaging in more patient-centred practice.
Conclusions
There has been a change in the symptoms that patients undergoing chemotherapy find most bothersome. While nausea, fatigue and general weakness remain common, the effect on family or partner was rated as the most severe, with fear of the future, not knowing what will happen, and putting my life on hold also major issues.
Data availability
Data will be shared on reasonable request to the corresponding author.
Code availability
Not applicable.
References
Coates A, Abraham S, Kaye SB et al (1983) On the receiving end—patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol 19:203–208
Griffin AM, Butow PN, Coates AS et al (1996) On the receiving end. V: patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol 7:189–195
Carelle N, Piotto E, Bellanger A et al (2002) Changing patient perceptions of the side effects of cancer chemotherapy. Cancer 95:155–163
Tran Y, Lamprell K, Nic Giolla Easpaig B et al (2019) What information do patients want across their cancer journeys? A network analysis of cancer patients' information needs. Cancer Med 8:155–164
Singh S, Butow P, Charles M, Tattersall MH (2010) Shared decision making in oncology: assessing oncologist behaviour in consultations in which adjuvant therapy is considered after primary surgical treatment. Health Expect 13:244–257
Lowy DR, Collins FS (2016) Aiming high—changing the trajectory for cancer. N Engl J Med 374:1901–1904
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Colinet B, Jacot W, Bertrand D et al (2005) A new simplified comorbidity score as a prognostic factor in non-small-cell lung cancer patients: description and comparison with the Charlson's index. Br J Cancer 93:1098–1105
Cella D, Tulsky DS, Gray G et al (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11:10
Yellen SB, Cella DF, Webster K et al (1997) Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manag 13:63–74
Goldberg DP. A user's guide to the General Health Questionnaire. In NFER-Nelson 1991
Rabin R, de Charro F (2001) EQ-5D: a measure of health status from the EuroQol Group. Ann Med 33:337–343
Ng TL, Hutton B, Clemons M (2015) Chemotherapy-induced nausea and vomiting: time for more emphasis on nausea? Oncologist 20:576–583
Vardy JL, Dhillon HM, Pond GR et al (2016) Fatigue in people with localized colorectal cancer who do and do not receive chemotherapy: a longitudinal prospective study. Ann Oncol 27:1761–1767
Goedendorp MM, Andrykowski MA, Donovan KA et al (2012) Prolonged impact of chemotherapy on fatigue in breast cancer survivors: a longitudinal comparison with radiotherapy-treated breast cancer survivors and noncancer controls. Cancer 118:3833–3841
Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB (2010) Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer 116:5740–5748
Koedoot CG, Oort FJ, de Haan RJ et al (2004) The content and amount of information given by medical oncologists when telling patients with advanced cancer what their treatment options are. Palliative chemotherapy and watchful-waiting. Eur J Cancer 40:225–235
Basch E, Deal AM, Kris MG et al (2016) Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 34:557–565
Detmar SB, Muller MJ, Schornagel JH et al (2002) Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA 288:3027–3034
Hoffman KE, McCarthy EP, Recklitis CJ, Ng AK (2009) Psychological distress in long-term survivors of adult-onset cancer: results from a national survey. Arch Intern Med 169:1274–1281
Butow P, Sharpe L, Thewes B et al (2018) Fear of cancer recurrence: a practical guide for clinicians. Oncology (Williston Park) 32:32–38
Macquart-Moulin G, Viens P, Bouscary ML et al (1997) Discordance between physicians' estimations and breast cancer patients' self-assessment of side-effects of chemotherapy: an issue for quality of care. Br J Cancer 76:1640–1645
Fromme EK, Eilers KM, Mori M et al (2004) How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol 22:3485–3490
Basch E, Iasonos A, McDonough T et al (2006) Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol 7:903–909
Acknowledgements
In memoriam
The team dedicate this study to Professor Martin HN Tattersall and Mrs Rhonda Devine, both deceased. Prof Tattersall was a medical oncologist who we had the honour of working with. His exceptional career placed patients at the centre of cancer care and included pioneering research into evaluating patient perspectives on cancer care, psycho-oncology, improving doctor-patient communication, and shared decision-making. Mrs Rhonda Devine was a cancer nurse consultant and clinical trials nurse we were privileged to work with. She was the living embodiment of and fierce advocate for patient-centred cancer care. Both improved the lives of people living with cancer either directly through the care they delivered or the implementation of the research they undertook.
Thanks to Shirley Lundie-Jenkins and Felicity Leslie who conducted some of the patient interviews.
Thanks to Dr Ian Tannock who reviewed the manuscript and made helpful suggestions.
We would also like to acknowledge the patients, oncology doctors and nurses from all three sites who completed the questionnaires.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Author notes
Rhonda Devine and Martin H. N. Tattersall is deceased. This paper are dedicated to his/her memory.
Contributions
JV, HD and MHNT conceived the study and JV and HD developed the methodology. AW, IK, CO, AE, RD and CR contributed to data collection and data management. JV and HD provided supervision for the study and research team. AL performed the analysis. JV wrote the initial draft with support from AL. All living authors reviewed the manuscript, provided feedback and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was granted by each hospital institutional review board prior to commencement. Written informed consent was obtained from each subject.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 61 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vardy, J.L., Liew, A., Warby, A. et al. On the receiving end: have patient perceptions of the side-effects of cancer chemotherapy changed since the twentieth century?. Support Care Cancer 30, 3503–3512 (2022). https://doi.org/10.1007/s00520-022-06804-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-06804-1