Abstract
Background
Few mortality-scoring models are available for solid tumor patients who are predisposed to develop Escherichia coli–caused bloodstream infection (ECBSI). We aimed to develop a mortality-scoring model by using information from blood culture time to positivity (TTP) and other clinical variables.
Methods
A cohort of solid tumor patients who were admitted to hospital with ECBSI and received empirical antimicrobial therapy was enrolled. Survivors and non-survivors were compared to identify the risk factors of in-hospital mortality. Univariable and multivariable regression analyses were adopted to identify the mortality-associated predictors. Risk scores were assigned by weighting the regression coefficients with corresponding natural logarithm of the odds ratio for each predictor.
Results
Solid tumor patients with ECBSI were distributed in the development and validation groups, respectively. Six mortality-associated predictors were identified and included in the scoring model: acute respiratory distress (ARDS), TTP ≤ 8 h, inappropriate antibiotic therapy, blood transfusion, fever ≥ 39 °C, and metastasis. Prognostic scores were categorized into three groups that predicted mortality: low risk (< 10% mortality, 0–1 points), medium risk (10–20% mortality, 2 points), and high risk (> 20% mortality, ≥ 3 points). The TTP-incorporated scoring model showed excellent discrimination and calibration for both groups, with AUC being 0.833 vs 0.844, respectively, and no significant difference in the Hosmer–Lemeshow test (6.709, P = 0.48) and the chi-square test (6.993, P = 0.46). Youden index showed the best cutoff value of ≥ 3 with 76.11% sensitivity and 79.29% specificity. TTP-incorporated scoring model had higher AUC than no TTP-incorporated model (0.837 vs 0.817, P < 0.01).
Conclusions
Our TTP-incorporated scoring model was associated with improving capability in predicting ECBSI-related mortality. It can be a practical tool for clinicians to identify and manage bacteremic solid tumor patients with high risk of mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advances in surgery combined with targeted or chemotherapies have substantially improved the survival rate of solid tumor patients. However, because of baseline immunodeficiency, cytotoxic treatments, and frequent invasive procedures, solid tumor patients are at high risk of bloodstream infection (BSI). An estimated 5.5–16.4‰ of solid tumor patients developed BSI [1], which pose significant burden on healthcare institutions as well as patients’ families. In light of these considerations, accurate and timely prognostic assessment of the outcome of infected solid tumor patients is essential to triage them for appropriate care and treatment regimens.
Escherichia coli (EC) is the most common cause of Gram-negative BSI. The incidence of EC-caused BSI (ECBSI) is increasing in the UK and Europe [2,3,4]. EC was also the predominant pathogen of BSI in the Asia–Pacific region (26.0% overall) [5]. In China, EC ranked first in the top five bacteremic pathogens from 2011 to 2016 [6]. Multidrug-resistant EC (e.g., extended-spectrum-beta-lactamase, ESBL) has spread in recent decades, and became a major health problem worldwide. This problem is of particular concern among solid tumor patients with immunosuppressed status, who are at high risk of severe sepsis and with BSI-related mortality. Currently, treatment of ECBSI is still a challenge, which makes the morbidity and mortality in infected solid tumor patients high [1, 7].
Several scoring models were established to estimate the BSI-related mortality in infected patients. These scoring models generally adopt risk factors from clinical and laboratory variables. For example, Al-Hasan et al. established a mortality-predictive model by using the Pitt bacteremia score (PBS) together with risk factors including malignancy, liver cirrhosis, and non-urinary/CVC source of BSI [8]. This scoring model is applicable for Gram-negative BSI patients who received adequate empirical antimicrobial therapy. Yishu Tang et al. established a scoring model that is suitable to identify 30-day mortality in hematologically malignant (HM) patients with BSI [9]. Risk factors including in this model are relapsed or uncontrolled malignancy, use of vasopressors, fungemia, acute respiratory failure, and hyperbilirubinemia together with PBS > 3. Other studies about BSI-related mortality-predictive models involve critical patients, sepsis patients, and high-risk patients in the emergency department [10,11,12,13]. Obviously, these scoring models were established based on clinical data from relatively homogeneous patients’ population, and could not be applicable to solid tumor patients due to differences in infected pathogen spectrum, patient characteristics, and treatment preference. More importantly, recent studies have shown that blood culture time to positivity (TTP) represents a quantitative surrogate predictor of the severity of BSI. Shorter TTP is associated with poor outcome of patients infected with certain organisms (e.g., Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Candida species) [14,15,16,17,18]. We hypothesized that TTP may be a useful predictor to solid tumor patients with bacteremia, and may be incorporated into a scoring model for the prediction of mortality. Therefore, the objective of this study was to develop a TTP-incorporated scoring model to identify solid tumor patients at risk of ECBSI-caused mortality.
Methods
Setting and study design
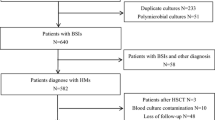
This cohort study was conducted in Tianjin Medical University Cancer Institute and Hospital, a 2400-bed medical center providing primary and tertiary care in northern China. The study was evaluated by the Ethics Committee of Tianjin Medical University and deemed exempt from a formal review as no personally identifiable information was collected. The requirement for informed consent from patients was also waived. All patients with blood cultures that yielded Escherichia coli between January 2013 and December 2018 were enrolled.
During the study period, the utilization of anti-infection therapies for solid tumor patients was performed according to the guidelines [19, 20]. For patients who had more than one positive culture with the same speciation and sensitivity, only the first one was counted. Clinical data on demographic characteristics; comorbid condition; and treatment course during hospitalization, discharge diagnosis, and outpatient follow-up were collected through electronic medical record review. Exclusion criteria for patients were (1) blood cultures’ contamination; (2) polymicrobial BSI (blood culture samples which showed different bacterial strains within 48 h); (3) a recurrent BSI occurring in the same patient; (4) incomplete clinical data including loss to follow-up; and (5) < 18 years old.
Solid tumor patients with ECBSI were assigned to the development group (two-thirds of the total number) and the validation group (one-third) with random distribution function of SPSS software. The primary outcome was in-hospital mortality after the onset of ECBSI. The survivors and non-survivors were compared to identify the potential risk factors for mortality. The development group was used to create mortality-scoring model, and scoring performance of the model was assessed by the validation group.
Microbiology analyses
Blood cultures were obtained by inoculation of blood samples into aerobic and anaerobic flasks. Blood samples were incubated in a BACTEC FX400 Automated Blood Culture System (Becton Dickinson, USA) in the clinical microbiology laboratory, and monitored for CO2 production every 10 min for 5 days. The time from blood culture inoculation to the detection of a positive signal was reported as TTP [14]. In patients with multiple sets of positive blood culture, the shortest TTP was used for analysis. All culture-positive blood samples were subcultured to identify bacterial isolates, according to the VITEK 2 automated microbiology system (bioMérieux, France) with conventional biochemical methods. All antibiotic susceptibilities were evaluated via minimal inhibitory concentration (MIC) according to the Clinical Laboratory Standard Institute (CLSI) criteria [21]. ESBL-EC was further confirmed by performing the double-disc synergy tests [22]. Escherichia coli ATCC25922 (negative control) and Klebsiella pneumoniae ATCC 700,603 (positive ESBL producer) were used as quality controls.
Variables and definitions
The following variables of each patient were collected at the onset of ECBSI: malignancy diagnosis, disease status, comorbidities, and antibiotic therapy. As previously described, ECBSI was defined as the isolation of Escherichia coli from at least one bottle of blood culture specimens from patients with compatible clinical signs or symptoms [1]. The date of the first positive blood culture was regarded as the onset date of BSI. Disease status was assessed by the most recently available tumor biopsy and categorized as remission, relapsed, and uncontrolled malignancy, following the previous definition [19]. Neutropenia was defined as an absolute neutrophil count (ANC) of < 500 cells/mm3 [23]. Acute respiratory distress syndrome (ARDS) was defined as previously described [23]. Inappropriate antibiotic therapy was defined as the empiric administration of antimicrobial agents that were ineffective against the causative microorganism either in vivo or in vitro [24]. In-hospital mortality was defined as death by any cause within the first 30 days after the onset of BSI during hospitalization [8].
Statistical analyses and model setup
Median values and interquartile range (IQR) were calculated for continuous variables, and percentages were used for categorical variables. The Mann–Whitney U test was used to compare continuous variables, and the categorical variables were analyzed with chi-square test. The cutoff values were set according to clinical practice or laboratory references. Potential risk factors for in-hospital mortality with P < 0.05 on univariable regression analysis were further investigated by multivariable regression analysis, using a backward selection method. Significant mortality-associated risk factors were assigned weighted points that were proportional to their β regression coefficient values. The risk scores were calculated for each patient. Patients were categorized at deciles of risk score, and then divided into three catalogues: low risk (< 10% predicted mortality), medium risk (10–20% predicted mortality), and high risk (> 20% predicted mortality). The predictive mortality was calculated for each risk catalogue, and the discriminatory ability of the scoring model was assessed by the area under the receiver operating characteristic (ROC) curve (AUC). Following the model establishment, validation was performed by using variables from the validation group. The difference of AUC between the two groups was compared using the chi-square test. The positive predictive value (PPV), negative predictive value (NPV), predictive sensitivity, and specificity were calculated at different cutoff values. The best cutoff value for mortality-risk stratification was determined based on Youden index statistics. Data were analyzed with SPSS software. All P values are two-sided, and a P-value < 0.05 was considered statistically significant.
Results
Clinical data of 535 solid tumor patients with ECBSI were collected. Of those, 23 recurrent episodes and three incomplete data of cases were excluded from the analysis. Thus, a total of 509 solid tumor patients with a first episode of ECBSI were finally enrolled. Demographic and clinical characteristics of solid tumor patients are shown (Table 1). Median age was 61 years and 30-day in-hospital mortality was 22.2% for overall patients. There was no significant difference between the two groups in all the characteristics under investigation (Table 1), indicating the grouping was random and even.
Variables from the development group were used to establish a risk-scoring model for predicting the mortality. Results from univariable regression analysis showed that fever ≥ 39 °C, inappropriate antibiotic therapy, metastasis, central line, blood transfusion, hypertension, ARDS at admission, and TTP ≤ 8 h were potential risk factors related to the mortality (Table 2). The multivariable regression model, which incorporates all potential risk factors from the univariable regression analysis, identified six predominant predictors for in-hospital mortality: fever ≥ 39 °C (OR = 2.93, 95% CI = 1.388–6.185, p = 0.005), inappropriate antibiotic therapy (OR = 3.636, 95% CI = 1.895–6.975, p < 0.001), metastasis (OR = 2.972, 95% CI = 1.443–6.119, p = 0.003), ARDS (OR = 10.159, 95% CI = 2.678–38.529, p = 0.001), blood transfusion (OR = 2.884, 95% CI = 1.511–5.505, p = 0.001), and TTP ≤ 8 h (OR = 2.64, 95% CI = 1.28–5.444, p = 0.009) (Table 2). These six independent predictors were then combined to further calculate the risk score of mortality in solid tumor patients with ECBSI.
Point values assigned to each predictor within the established TTP-incorporated and the no TTP-incorporated (NTTP) scoring models are shown (Table 3). In development group, the predicted mortality for low-risk, medium-risk, and high-risk categories by TTP-incorporated model was 4.38%, 15.39%, and 51.77%, respectively, while those for validation group were 3.72%, 13.88%, and 50.09%, respectively (Fig. 1). No difference for the predicted mortality was observed between the two groups. However, this prediction by two models was distinct in both groups with a proportion of patients in low-risk, medium-risk, and high-risk groups of 4.12%, 14.8%, and 51.46% (for TTP model), and 7.83%, 17.97%, and 44.01% (for NTTP model), respectively (Fig. S1). The TTP model showed excellent discrimination, with AUC being 0.833 (95% CI = 0.779–0.887) in development group versus 0.844 (95% CI = 0.774–0.913) in validation group (Fig. 2). Similar discrimination in two groups was found with AUC being 0.837 (95%CI = 0.795–0.880) by TTP model versus 0.817 (95%CI = 0.774–0.861) by NTTP model (Fig. S2). The model also had good calibration in both groups, without difference after the Hosmer–Lemeshow test (6.709, P = 0.48) and the chi-square test (6.993, P = 0.46). In both development and validation groups, our model showed reliable and consistent increase in mortality with increasing score (Fig. S3). Predictive sensitivity and specificity, PPV and NPV at different thresholds of the two scoring models are shown (Table 4). The Youden index indicated that the TTP-incorporated scoring model performed best at a cutoff value of ≥ 3 points (Table 4, Table S1), whereas the NTTP scoring model performed best at a cutoff value of ≥ 2 points (Table 4). Prediction sensitivity and NPV increased along with the decrease of cutoff values, indicating our scoring model provides highly diagnostic accuracy.
Discussion
In this study, we developed a new TTP-incorporated scoring model to predict the bacteremia-associated mortality, and evaluated its capability of risk stratification among solid tumor patients. To our knowledge, this is the first mortality-scoring model established for solid tumor patients with ECBSI. Although one study reported a TTP-incorporated scoring model [25], it was designed to stratify the risk of nontyphoid Salmonella-caused vascular infection (NTSVI). As nontyphoid Salmonella bacteremia is not frequent, the TTP-NTSVI scoring model is not applicable to bacteremic solid tumor patients.
The requirement to stratify solid tumor patients at risk of in-hospital mortality is particularly relevant to those with bloodstream infections. Our established scoring model utilizing TTP and reliable predictors based on modern epidemiology was associated with improved mortality-prediction performance. This performance was well-evidenced both in the development and validation groups, as the prediction sensitivity of our scoring model was improved with the decrease of cutoff values (Table 4). Although at a cutoff value of 3 the prediction sensitivity for both groups was relatively low (76.71% and 75%, respectively), high prediction specificity improved the targeting of solid tumor patients with high mortality risk. Our model showed the predicted mortality increased along with increasing score (Fig. S3). This indicates that solid tumor patients enrolled in this study were likely to have a mild infection and a low mortality at a scale of 0 to 1. If the score increased, the patients might have a severe infection and a high risk of mortality. Therefore, our new TTP-incorporated scoring model could be used as a screening tool to quickly identify high-risk solid tumor patients who may benefit from early intervention.
TTP is a newly developed laboratory indicator. Based on the assessment of initial bacterial inoculum in cultured blood, it was proved to be a useful predictor for the severity of bacteremia, and associated with clinical outcomes of patients with S. aureus, Streptococcus pneumoniae, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa bacteremia [14,15,16,17,18, 26]. However, prior to this study, the association of shorter TTP with higher risk of mortality was mainly reported in non-tumor patients with Klebsiella pneumoniae bacteremia [26]. In this study, we showed that TTP was a dominant predictor for in-hospital mortality in solid tumor patients with ECBSI (Table 2). Our study therefore supports the idea that TTP is a power to provide both prognostic and diagnostic information for physicians in treating solid tumor patients with bacteremia.
In spite of the updated guidelines for the management of bacteremic patients, in-hospital mortality due to BSI in cancer patients remains high. We found in this study that inappropriate antibiotic therapy was associated with high mortality of solid tumor patients with ECBSI (Table 2). Reducing mortality by the rapid initiation of empirical antibiotic therapy for cancer patients is undisputed. However, the frequent cases in the clinic are in vitro susceptibility tests were sensitive, whereas the antibiotic application in solid tumor patients with ECBSI was ineffective. This implies that there might have certain pathophysiological variables in infected solid tumor patients, especially those in advanced stages. Therefore, it is necessary to consider the optimal dosing and tailored individual regimens of antimicrobials for tumor patients with ECBSI.
ARDS is more often fatal in infected patients [27]. In this study, the mortality of bacteremic solid tumor patients with ARDS was accounted for 15.1% (Table 2). This may be due to solid tumor patients undergoing extensive tumor resections, in particular involving the respiratory and gastrointestinal tracts, who are at greater risk of developing postoperative nosocomial infections. In addition, they may have severe acute lung injury related to chemotherapeutic agents and radiation [1]. Given the high mortality associated with ARDS, efforts should be taken to prevent ARDS in solid tumor patients with ECBSI. Early utilization of high-resolution chest CT-scan, serological tests such as galactomannan antigen and bronchoscopy with bronchodilator lavage, could reduce the in-hospital mortality of solid tumor patients with ARDS.
It is a remarkable fact that the blood transfusion as a mortality predictor in bacteremic solid tumor patients has not been reported (Table 2 and Table 3). Anemia is frequently observed in cancer patients, either due to chronic illness or active bleeding. Blood transfusion supplements blood volume and improves microcirculation, but the increased plasma protein may promote coagulation. One study reported transfusion of blood components actually led to worse outcomes in tumor patients [1]. Thus, blood transfusion should be regarded as personalized medicine, carefully considering the bacteremic status of solid tumor patient in order to reduce the in-hospital mortality.
There are two limitations in this study. First, since the current study was performed at a single center in China, liver cancer comprises the most common solid tumor, followed by pancreatic cancer. Liver cancer is less common in the North America and Europe. The difference in expected tumor type would impact the predictive power of this model outside of China. Second, no uniform standard is available for variables in the predictive model and it needs further validation by multiple center research in the future.
Conclusions
Unlike the HM patients with bacteremia, among those ICU admission, ESBL and neutropenia are the frequent predictors of ECBSI-caused mortality [9, 24]. In bacteremic solid tumor patients, TTP ≤ 8 h, inappropriate antibiotic therapy, ARDS at admission, blood transfusion, fever ≥ 39 °C, and metastasis are predominant predictors associated with ECBSI-caused mortality. We developed a mortality-risk-scoring model that incorporates TTP with other five reliable predictors in solid tumor patients with ECBSI. Our TTP-incorporated scoring model improved the mortality-predictive capability in solid tumor patients with bacteremia. It can be a practical tool for clinicians to identify and manage bacteremic solid tumor patients with high risk of mortality.
Availability of data and material
The data of this study are available from the corresponding author upon request.
Code availability
N/A.
Abbreviations
- TTP:
-
Time to positivity
- EC:
-
Escherichia coli
- BSI:
-
Bloodstream infection
- ECBSI:
-
Escherichia coli–Caused bloodstream infection
- ARDS:
-
Acute respiratory distress
- IQR:
-
Interquartile range
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under a ROC curve
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive values
- HM:
-
Hematological malignant
- MIC:
-
Minimum inhibitory concentration
- CLSI:
-
Clinical and Laboratory Standards Institute
References
Gudiol C, Aguado JM, Carratala J (2016) Bloodstream infections in patients with solid tumors. Virulence 7(3):298–308
Blot K, Hammami N, Blot S, Vogelaers D, Lambert ML (2019) Increasing burden of Escherichia coli, Klebsiella pneumoniae, and Enterococcus faecium in hospital-acquired bloodstream infections (2000–2014): a national dynamic cohort study. Infect Control Hosp Epidemiol 40(6):705–709
van der Mee-Marquet NL, Blanc DS, Gbaguidi-Haore H, Borges SDS, Viboud Q, Bertrand X et al (2015) Marked increase in incidence for bloodstream infections due to Escherichia coli, a side effect of previous antibiotic therapy in the elderly. Front Microbiol 6:646
Buetti N, Atkinson A, Marschall J, Kronenberg A (2017) Incidence of bloodstream infections: a nationwide surveillance of acute care hospitals in Switzerland 2008–2014. BMJ Open 7(3):e013665
Diekema DJ, Hsueh PR, Mendes RE, Pfaller MA, Rolston KV, Sader HS et al (2019) The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother 63(7):e00355-e419
Wang XJ, Zhao CJ, Li HN, Chen HB, Jin LY, Wang ZW et al (2018) Microbiological profiles of pathogens causing nosocomial bacteremia in 2011, 2013 and 2016. Sheng Wu Gong Cheng Xue Bao 34(8):1205–1217
Gudiol C, Tubau F, Calatayud L, Garcia-Vidal C, Cisnal M, Sánchez-Ortega I et al (2011) Bacteraemia due to multidrug-resistant Gram-negative bacilli in cancer patients: risk factors, antibiotic therapy and outcomes. J Antimicrob Chemother 66(3):657–663
Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM (2013) Predictive scoring model of mortality in Gram-negative bloodstream infection. Clin Microbiol Infect 19(10):948–954
Tang YS, Cheng Q, Yang Q, Liu J, Zhang D, Cao W et al (2018) Prognostic factors and scoring model of hematological malignancies patients with bloodstream infections. Infection 46(4):513–521
LeGall JR, Loirat P, Alpérovitch A (1986) APACHE II–a severity of disease classification system. Crit Care Med 14(8):754–755
Finkelsztein EJ, Jones DS, Ma KC, Pabón MA, Delgado T, Nakahira K et al (2017) Comparison of qSOFA and SIRS for predicting adverse outcomes of patients with suspicion of sepsis outside the intensive care unit. Crit Care 21(1):73
Shapiro NI, Wolfe RE, Moore RB, Smith E, Burdick E, Bates DW (2003) Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med 31(3):670–675
Tumbarello M, Trecarichi EM, Caira M, Candoni A, Pastore D, Cattaneo C et al (2012) Derivation and validation of a scoring system to identify patients with bacteremia and hematological malignancies at higher risk for mortality. PloS One 7(12):e51612
Zhang Q, Li D, Bai CS, Zhang WF, Zheng S, Zhang P et al (2016) Clinical prognostic factors for time to positivity in cancer patients with bloodstream infections. Infection 44:583–588
Peralta G, Roiz MP, Sánchez MB, Garrido JC, Ceballos B, Rodríguez-Lera MJ et al (2007) Time-to-positivity in patients with Escherichia coli bacteraemia. Clin Microbiol Infect 13(11):1077–1082
Marra AR, Edmond MB, Forbes BA, Wenzel RP, Bearman GML (2006) Time to blood culture positivity as a predictor of clinical outcome of Staphylococcus aureus bloodstream infection. J Clin Microbiol 44(4):1342–1346
Willmann M, Kuebart I, Vogel W, Flesch I, Markert U, Marschal M et al (2013) Time to positivity as prognostic tool in patients with Pseudomonas aeruginosa bloodstream infection. J Infect 67(5):416–423
Nunes CZ, Marra AR, Edmond MB, Victor EDS, Pereira CAP (2013) Time to blood culture positivity as a predictor of clinical outcome in patients with Candida albicans bloodstream infection. BMC Infect Dis 13:486
de Naurois J, Novitzky-Basso I, Gill MJ, Marti FM, Cullen MH, Roila F (2010) Management of febrile neutropenia: ESMO Clinical Practice Guidelines. Ann Oncol 21 Suppl 5:v252-256
Flowers CR, Seidenfeld J, Bow EJ, Karten C, Gleason C, Hawley DK et al (2013) Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 31(6):794–810
Institute CaLS (2011) Performance standards for antimicrobial susceptibility testing: twenty first informational supplement M100-S21. Wayne, PA, USA
Institute CaLS (2010) Performance standards for antimicrobial susceptibility testing: twenty first informational supplement M100-S20. Wayne, PA, USA
Hall KK, Lyman JA (2006) Updated review of blood culture contamination. Clin Microbiol Rev 19:788–802
Marin M, Gudiol C, Ardanuy C, Garcia-Vidal C, Jimenez L, Domingo-Domenech E et al (2015) Factors influencing mortality in neutropenic patients with haematologic malignancies or solid tumours with bloodstream infection. Clin Microbiol Infect 21:583–590
Lin JJ, Weng TH, Tseng WP, Chen SY, Fu CM, Lin HW et al (2018) Utility of a blood culture time to positivity-incorporated scoring model in predicting vascular infections in adults with nontyphoid Salmonella bacteremia. J Microbiol Immunol Infect 51(5):652–658
Liao CH, Lai CC, Hsu MS, Huang YT, Chu FY, Hsu HS et al (2009) Correlation between time to positivity of blood cultures with clinical presentation and outcomes in patients with Klebsiella pneumoniae bacteraemia: prospective cohort study. Clin Microbiol Infect 15:1119–1125
Doern GV, Carroll KC, Diekema DJ, Garey KW, Rupp ME, Weinstein MP et al (2019) Practical guidance for clinical microbiology laboratories: a comprehensive update on the problem of blood culture contamination and a discussion of methods for addressing the problem. Clin Microbiol Rev 33(1):e00009-19
Funding
This work was supported by the Key Research and Development Program of Tianjin Municipal Science and Technology Commission (20YFZCSY00450), Chinese Pharmaceutical Association-Yiling Biomedical Innovation Fund Project (CPAYLJ202003), and Nankai-Cangzhou NCC Fund Project (NCC2020PY20).
Author information
Authors and Affiliations
Contributions
All authors contributed to data analysis, drafting, or revising the article; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was discussed and approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital.
Consent to participate
The requirement for informed consent from patients was waived by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital.
Consent for publication
All authors reviewed the final version of this manuscript, and agree to submit it.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Q., Gao, HY., Li, D. et al. A TTP-incorporated scoring model for predicting mortality of solid tumor patients with bloodstream infection caused by Escherichia coli. Support Care Cancer 30, 413–421 (2022). https://doi.org/10.1007/s00520-021-06442-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06442-z