Abstract
Purpose
Physical activity (PA) is recommended to improve advanced cancer patients’ (ACP) physical functioning, fatigue, and quality of life. Yet, little is known about ACPs’ attitude towards PA and its influence on fatigue and depressiveness over a longer period. This prospective, non-interventional cohort study examined ACPs’ fatigue, depression, motivation, and barriers towards PA before and after 12 months of treatment among ACP
Methods
Outpatients with incurable cancer receiving treatment at a German Comprehensive Cancer Center reporting moderate/severe weakness/tiredness during self-assessment via MIDOS II were enrolled. Fatigue (FACT-F), depression (PHQ-8), cancer-related parameters, self-assessed PA behavior, motivation for and barriers against PA were evaluated (T0). Follow-up data was acquired after 12 months (T1) using the same questionnaire.
Results
At follow-up, fatigue (p=0.017) and depressiveness (p=0.015) had increased in clinical relevant extent. Physically active ACP did not show significant progress of FACT-F (p=0.836) or PHQ-8 (p=0.799). Patient-reported barriers towards PA remained stable. Logistic regression analyses identified motivation as a positive predictor for PA at both time points (T0, β=2.152, p=0.017; T1, β =2.264, p=0.009). Clinically relevant depression was a negative predictor for PA at T0 and T1 (T0, β=−3.187, p=0.044; T1, β=−3.521, p=0.041).
Conclusion
Our findings emphasize the importance of psychological conditions in physical activity behavior of ACP. Since psychological conditions seem to worsen over time, early integration of treatment is necessary. By combining therapy approaches of cognitive behavioral therapy and exercise in interdisciplinary care programs, the two treatment options might reinforce each other and sustainably improve ACPs’ fatigue, physical functioning, and QoL.
Trial registration
German Register of Clinical Trials, DRKS00012514, registration date: 30.05.2017
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Therapy improvements achieved by cancer research and early integration of palliative and supportive care lead to a longer survival period in patients with incurable cancer (advanced cancer patients; ACP) [1, 2]. As a result, the quality of life of patients and the factors influencing it are increasingly moving into the focus of optimized care. Especially, cancer-related fatigue (CRF) is one of the most distressing symptoms in ACP [3, 4]. The presence of weakness and tiredness cannot be improved by rest and is more agonizing than sleepiness experienced by healthy individuals, which indicates the syndrome of CRF [5,6,7]. The National Comprehensive Cancer Network guidelines [5] recommend several non-pharmacological interventions including physical activity and psychosocial therapies as treatment of CRF. Several studies and meta-analyses showed a promising way through exercise to reduce fatigue and improve ACPs’ physical functioning and maintain their independence [5, 8,9,10,11]. Recently published data [12] suggests cognitive behavioral therapy as a helpful treatment approach for the same purpose. The American Cancer Society’s latest guidelines [13] recommend exercise for ACP adjusted to their individual physical abilities. In contrast, only less than 30% manage to be physically active at all [14,15,16]. While there is some knowledge regarding cancer survivor’s barriers of physical activity [17,18,19,20,21], only little is known about barriers towards exercise in ACP. Studies [14, 22,23,24] examined barriers towards physical activity among ACP or a mixed cohort of cancer survivors and ACP. They found lack of motivation, active systemic cancer treatment and its side effects, overwhelming fatigue, and psychosocial factors (e.g., depression, no access to facilities, bad weather) as possible reasons for patient’s inactivity. In addition to these qualitative studies, supplementary quantitative data would help to improve the current state of research. Furthermore, data about fatigue and its accompanying symptoms in ACP or cancer survivors for a longer duration of time is scarce. Few surveys indicate that CRF might increase over time [25,26,27,28]. Since there is a steady impact of cancer therapy on patients’ quality of life [29], more information on changes in barriers related to cancer treatment might help in developing suitable exercise programs. The study objective was to compare self-reported fatigue, depression, motivation, and barriers to exercise before and after 12 months of cancer treatment among ACPs. As secondary objective, we aimed to explore the differences in predictors of self-assessed physical activity between before and after 1 year.
Methods
Study protocol and patient recruitment
The study was conducted in a large outpatient unit of a German Comprehensive Cancer Center and had a prospective, non-interventional design. All outpatients of the cancer center quarterly answered a validated self-assessment instrument (MIDOS II (the new minimal documentation system) [30]) for the purpose of measuring ACPs’ physical and cognitive symptom burden. The MIDOS II is a German variation of the Edmonton Symptom Assessment Scale (ESAS) [30]. Symptoms are described in a 4-point Likert scale with following options: none, low, moderate, and severe. Patients suffering from moderate to severe tiredness and/or weakness were regarded as eligible for the study. Additionally, participants had to meet the following inclusion criteria: age above 18 years, histologically confirmed cancer with UICC stage IV, capability to understand and answer the German questionnaire, and absence of serious pulmonic or cardiac comorbidities.
Patients fulfilling the eligibility criteria and agreed to participate obtained an information sheet. An in-person paper-based questionnaire was completed on the same day of signing the consent form (T0). All patients underwent some kind of cancer treatment during T0. After 12 months, the paper-based follow-up questionnaire was sent by mail and patients were asked to return the completed questionnaire at their next appointment (T1).
Questionnaire design
The applied questionnaire consisted of 64 items including the Functional Assessment of Cancer Therapy Fatigue (FACT-F, [31,32,33,34]); the Patient Health Questionnaire depression scale (PHQ8, 8 items [35,36,37]); demographic data (6 items); anticipated psychological, physical, and social barriers (5-, 12-, and 8 items); and questions regarding participants’ physical activity behavior (6 items). Detailed information on the questionnaire structure has been published previously [14].
The FACT-F is a 13-item questionnaire measuring self-reported fatigue. Therefore, a 5-point Likert scale is used. Scores range from 0 (high symptom burden) to 52 (low symptom burden), with ≤ 34 points indicating a pathological FACT-F Score [34]. The PHQ8 is a common tool for measurement of depressiveness. It is based on 8 items with a score of 0 to 3 points. The PHQ-8 score ranges from 0 to 24 points, a score ≥ 10 points to clinically relevant depression [36].
For information on demographics, patients were asked for marital status, living situation, number of children nationality, and educational attainment.
Patient-reported motivation towards physical activity, their activity before cancer diagnosis, their interest in attending an exercise program, and their knowledge about exercise and QoL were measured on a 5-point Likert scale (0= not at all; 1= a little bit; 2= somewhat; 3= quite a bit; 4= very much) [17, 38, 39]. For calculation of relative risk, the answers “not at all”/”a little bit” and “somewhat”/“quite a bit”/“very much” of the 5-pont Likert scale were combined.
To assess subjective physical activity, patients could cross mark their current activity level and add frequency (1= 1 time; 2= 2–3 times; 3= more than 3 times per week) and intensity (1=light, 2=moderate, 2= severe); or in case they have a workout partner, whether their life partner was working out and if they were participants in an exercise program.
For evaluation of anticipated physical barriers in ACP [17, 18, 39], several somatic symptoms (weakness, pain, shortness of breath, tiredness, vomiting/nausea, and joint complaints) were selectable. Furthermore, patients could answer “yes” or “no” questions regarding if they felt weakened due to cancer therapy, had been hospitalized frequently, are afraid of damage from exercising, and if their still smoking.
For assessment of possible social barriers [24, 39], patients could choose between “no local physiotherapist,” “no payed transport,” “missing prescription,” “lack of time,” “stressful daily life,” “too many other commitments,” “bad weather,” and “PA is too expensive.” If applicable, multiple selections of social barriers were possible.
Patient-related data
Patient-related data including gender, age, comorbidities, tumor entity, type of cancer treatment, previous palliative therapy lines, disease progress over time, and performance status were assessed by patient file revision.
Statistical methods
For descriptive statistics presented in Table 1 and Fig. 1, median, mean values, and standard deviations were generated by SPSS (Version 23). For group comparisons, between the study population at baseline (T0, n=63) and after 12 months (T1, n=63) shown in Table 1, we used the Fisher’s exact test on nominally scaled variables. For comparison in means, we used the paired t test on normally distributed variables, while the non-parametric Wilcoxon test was used on non-normally distributed variables. For comparison of means in fatigue, depression, and motivation at baseline and after 12 months, as well as group comparisons between physically active and physically inactive patients at T0 and T1 (Fig. 2), the non-parametric Wilcoxon test was used. Further group comparisons (e.g., physically active vs. physically inactive patients) were performed depending on sample sizes and scale level (Fisher’s exact test, χ2-Test, Mann-Whitney U Test, independent t test). In order to measure the strength of relation between barriers towards exercise at baseline and after 12 months (Table 2), relative risks (RR) and the 95% confidential interval for each barrier were calculated. Fisher’s exact test was used for calculation of p values. To examine differences in predictors for physical activity and motivation for exercise at baseline and at follow-up (Table 3), binary logistic regression analyses were performed. In both models, the same independent variables were used. For all tests, we defined a significance level of p<0.05. Calculation of optimal sample size was performed by using G*Power3 [40]. Regarding the outcome variables fatigue and depressiveness at T0/T1, assuming a small effect size according to Cohen [41] and a power of β=0.8, a sample size of N=33 was determined.
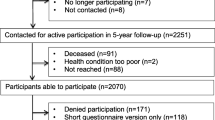
Flowchart of patient enrollmenta. For preselection the validated MIDOS II [31] was used; patients indicating moderate to severe tiredness/weakness were considered as eligible
Results
During the recruitment period from May 2017 to August 2018, N=1362 patients completed the symptom-assessment via MIDOS II [30]. Of those, n=725 (53.3%) questionnaires indicated moderate to severe tiredness and/or weakness. After identification of duplicates (n=285), 440 patients were eligible for study participation [14]. One hundred forty-one patients gave consent and answered the baseline questionnaire. Follow-up was answered by 63 participants (response rate: 44.7%) (Fig. 1). Patients who answered the questionnaire at both requested time points form in the final study cohort were referred to as “follow-up group.” Assessing the group of participants, which did not complete the follow-up (drop-out group), 46 (59.0%) patients deceased within 1 year, eight (10.3%) patients were lost, and 24 (30.8%) individuals did not answer the survey.
Patient population
Characteristics of follow-up group at baseline and 12 months questionnaire are presented in Table 1. At baseline, 14 patients classified themselves as being physically active. After 12 months, this number increased to 16 participants. In addition to the information in Table 1, the follow-up group (n=63) and deceased patients (n=46) were compared regarding physical activity patterns, symptom burden of CRF and depression, mentioned barriers towards physical activity, and cancer-related parameters. Deceased patients reported significantly more weakness (follow-up patients, n=33 (52.4%); deceased patients, n=34 (71.7%); p=0.029). The follow-up group had a better ECOG performance status (p=0.049) and less patients received chemotherapy (follow-up group, n=35 (55.6%); deceased patients, n=36 (78.3%); p=0.016). No further differences were detected. Mean time before death of deceased patients was 6.22 (±3.3, 1–12) months.
Patient reported outcomes: barriers towards exercise at baseline and after 12 months
Compared to baseline, two more patients claimed to be physically active at follow-up [T0, n=14 (22.2%); T1, n=16 (25.4%) [RR 1.04; 95 %; CI 0.86–1.27]). Patient-reported barriers towards physical activity at baseline and after 12 months are presented in Table 2. At baseline “sleep disturbance” was the most frequent reported barrier (n=51, 81.0%). “Feeling weakened due to cancer therapy” was most commonly chosen barrier in follow-up (n=51, 81%). Calculation of relative risk showed improvement in records of all regarded physical barriers (except prevalence of “sleep disturbance” and “nausea/vomiting”). Group comparisons between baseline and follow-up revealed no statistically significant differences between the listed barriers. At baseline, patients chose 0.27 out of the eight provided social barriers. The follow-up revealed a mean of 0.34 in the chosen number of social barriers. Neither comparisons in mean (p=0.828) nor group comparisons concerning each social barrier (except for “no local physiotherapist” [RR 2.15 95 %; CI 1.77–2.60; p=0.006]) showed remarkable discrepancies between the two dates of questionnaire. After 12 months, fewer patients had an ECOG performance status of 0 or I [RR 1.27 95%CI 1.06–1.51; p=0.011]. Mean number of palliative chemotherapy line was significantly higher at T1 (T0, 1.1(± 1.4); T1, 2.0 (±1.8); p<0.001)
Changes of means in fatigue, depressiveness, and self-reported motivation
Comparisons in means regarding fatigue at baseline (T0) and after 12 months of cancer treatment (T1) are reported in Fig. 2a. A significant decrease of FACT-F score (3.5 points on average, p=0.017) among the study population could be detected. Subgroup analyses in means of FACT-F score showed a significant improvement in patients physical inactivity (3.9 points on average; p=0.007), while means of FACT-F score did not change significantly in self-assessed physically active participants (0.3 points on average, p=0.836). There were no significant differences in numbers of patients diagnosed with clinically relevant fatigue (pathological FACT-F score; ≤ 34 points [34]; T0, n= 41, 65.1%; T1, n=50, 79.4%; p=0.111). In the subgroup of physically inactive patients, substantially more participants suffered from clinically relevant fatigue (T0, n=30, 47.6%; T1, n=38, 60.3%; p=0.044). As presented in Fig. 2b, PHQ8 scores raised significantly (0.9 points on average, p=0.015) within ACP. Physically inactive participants had higher depression score at T1 (1.5 points on average, p=0.011). Physically active participants did not show any increase in PHQ8 scores (0.0 points on average, p=0.799). After 12 months of treatment, more patients could be diagnosed with clinically relevant depression [36] (PHQ8≥10; T0, n=19, 30.2%; T1, n=32, 50.8%; p= 0.029).
No statistically relevant increase or decrease could be identified in the patients’ self-reported motivation towards physical activity. Neither physically active nor physically inactive individuals had alterations relating to this variable. Physically active patients claimed to be more motivated for physical activity at any time (T0, p<0.001; T1, p<0.001).
Prediction of subjective physical activity
Binary regression analyses were performed in order to determine predictors of patient-reported physical activity at T0 and T1 (Table 3). Significant parameter regarding physical activity in physically active/inactive patients was included in the analysis. Both models were significant and showed strong goodness-of-fit (T0, R2= 0.719, f=1.60; T1, R2= 0.704, f= 1.54) according to Cohen [41]. The model demonstrated that 71.9% (T0) and 70.4% (T1) of physically active behavior could be explained by the independent variables. Motivation for physical activity (T0, β=2,152, p=0.017; T1, β=2.264, p=0.009) and clinically relevant depression (T0, β=−3.187 p=0.044; T1, β=−3.521, p=0.041) were significant predictors for physical activity at both time points. At baseline, breast cancer (β=5.345, p=0.018) and dyspnea (β=−6.558, p=0.016) were further identified predictors.
Discussion
This study aimed to reevaluate ACPs’ expression of fatigue, depression, and motivation and barriers for exercise after 12 months of cancer treatment. Possible changes in predictors of being physically active were determined by using logistic regression models.
Although patients were in an early stage of their palliative trajectory and over 90% had a good ECOG performance status, only one-fifth were physically active. Only two more participants engaged in physical activity at T1. Though reported barriers of physical activity did not increase in statistically significant value after 12 months, an increase in almost every reported barrier (except for sleep disturbance) was evident. Feeling weakened due to active systematic cancer treatment increased by 10% during 1 year of cancer treatment. The stated results also agree with the increase of ECOG and number of palliative chemotherapy lines. Various reasons can lead to this deterioration in treatment of ACP (e.g., not enough food intake, anorexia, reduced physical activity, tumor interaction). Additionally the direct influence of the palliative chemotherapy affecting different cytokines, lipolysis, proteolysis, and metabolism leads to adipose tissue and skeletal muscle mass loss [42]. Pathomechanisms may result in weakness and reduced physical functionality during advanced cancer treatment. Solheim et al. [43] measured change in weight, muscle mass, physical activity, and survival as secondary outcome of a multimodal exercise intervention in patients with incurable pancreatic and lung cancer suffering from cachexia. Though there were no significant differences between intervention and control group, muscle mass and weight remained stable among the intervention group. Muscle mass and weight remained stable among the intervention group. These results indicate that clinical deterioration of ACP could be slowed by multimodal interventions including exercise. Therefore, physical performance status should be evaluated on a regular basis during treatment of ACP. Standardized assessments of submaximal cardiorespiratory fitness and functional mobility might help to tailor physical activity programs to ACPs’ needs and abilities.
Our investigation is one of the studies that examined exercise in ACP with clinical depression as secondary outcome. Cormie et al. [44] and Tsianakas et al. [45] analyzed the effects of resistance and walking exercise in ACP on depression but did not find any significant results. Depression score stayed stable within little fluctuations. By contrast Pyszora et al. [11] presented significant findings related to decreased depression after a 2-week intervention of 30 min active exercise, myofascial release, and proprioceptive neuromuscular facilitation. Literature that highlights the influence of exercise on physical function/performance status, psychosocial symptoms, and quality of life in ACP are necessary in order to evolve solutions. Furthermore, Nipp et al. [46] demonstrated that the ongoing loss of weight/muscle mass and impairment in physical function is associated with a higher clinical depression.
The performed comparisons in mean of FACT-F score at T1 and T0 revealed an increase of FACT-F of 3.5 points on average. This points to a clinically relevant improvement of fatigue among our study cohort [31]. Only few studies have examined the course of fatigue during treatment of ACP and obtained different results. In 2016 Peters et al. [25] published a study that assessed the severity of CRF in patients undergoing palliative care over a mean time of 4.9 months. Fatigue remained steady among the population. Results of a prospective investigation by Verkissen et al. [29] showed that most symptoms including fatigue did not change significantly during treatment of ACP. In a cross-sectional study by Beernaert et al. [47], QoL was assessed in patients at three different treatment phases (curative, life-prolonging, and highly advanced). Participants in further stages of cancer treatment showed higher symptom burdens (especially fatigue) and lower QoL. In order to explain this deviation in findings, further longitudinal studies are required.
Currently, the impact of (patient-reported) physical activity on CRF in ACP is a highly discussed topic. The results of our non-interventional study suggest that CRF does not increase in patients that classified themselves as physically active. Several studies emphasized the positive effects of exercise on fatigue, QoL, and physical functioning [8, 9, 11]. A systematic review of physical activity interventions in ACP described contradictory results regarding the outcome of CRF [48]. The small sample size of our study could have lowered the power to identify significant differences in fatigue of physically active patients. In addition, our physical activity assessment grounded on subjective self-rated physical activity of our participants. Therefore, our results cannot be generalized, and more data on physical activity and CRF in ACP is necessary. Lately, Poort et al. [12] demonstrated in a randomized controlled trial that cognitive behavioral therapy improved physical functioning, QoL, and fatigue in a sample of ACP, but no statistically relevant alterations in patients receiving graded exercise therapy were detected. Referring to the close relationship of effective factors and physical activity (mentioned above), these two therapy approaches might reinforce each other as part of interdisciplinary programs.
The conducted analyses identified patient-reported motivation for physical activity and clinically relevant depression [36] as significant predictors for physical activity at both time points of survey. While motivation for physical activity was positively associated, depressiveness turned out to be a negative predictor. Baseline values of dyspnea (negative impact) and the tumor entity of breast cancer (positive impact) were substantial predictors for physical activity, which diminished over time. Some studies have examined predictors of this outcome in advanced cancer patients. Only few of them analyzed the course of predictors during cancer treatment. Ungar et al. [49] investigated physical activity enjoyment and self-efficacy in a mixed population of cancer survivors and ACP before and after a 4-week intervention and 10 weeks later. Self-efficacy and physical activity enjoyment at T0 were significantly associated with physical activity, whereas 10 weeks later only self-efficacy remained a considerable predictor. A systematic review of Ormel et al. [50] summarized predictors of adherence to physical activity in patients during and after cancer treatment. High motivation, high self-efficacy, and extensive exercise history correlated with better adherence to exercise programs. These findings indicate that performing physical activity strongly depends on patients’ psychological conditions such as motivation, self-efficacy, and depressiveness. Therefore, psychological counseling might be a promising way to promote physical activity in this particular patient population.
Limitations
There are several limitations to our study, which should be acknowledged. We measured the participants’ subjective perception of physical activity levels, and reports might differ from objectively assessed physical activity levels. Second, participants’ answers to the related questions were individual, and a generalization of our results is not possible. In order to develop suitable activity programs, we focused on participants’ attitude towards physical activity and its surrounding aspects. This inevitably includes their subjective opinions. This study had a monocentric setting and was performed in an outpatient care of a sizeable oncologic center in Germany. The different diagnoses among our study population may not be representative. The sample size of our study was relatively small. Therefore, our results only show tendencies, and more longitudinal analyses are required. Additionally, it should be considered that most of our participants were in an early stage of their disease. The majority of our cohort had a good performance status, and approximately 40% did not have palliative chemotherapy previous to answering the baseline questionnaire.
Conclusion
Cancer-related fatigue and depression increased in a clinically relevant dimension over the period of a year. Patients that rated themselves as physically active did not show significant progress in these symptoms. A motivated attitude and clinically relevant depression were identified as long-term predictors of subjective physical activity. Physical barriers were stated frequently but stayed stable at both measurements. Our findings emphasize the importance of psychological conditions and effective factors in physical activity behavior of ACP. Our results are in line with the latest interventional studies [12], highlighting that treatment programs for CRF should focus on early integration of both physical activity and psychological well-being. Interdisciplinary care programs that unite these two therapy concepts might help ACP not only in starting and maintaining physical activity but also in improving their psychological state of health. A sustainable decrease of fatigue and increase of patients’ QoL might be the promising outcome of this therapeutic approach.
References
Pinto BM, Trunzo JJ (2005) Health behaviors during and after a cancer diagnosis. Cancer 104:2614–2623
Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ (2010) Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363:733–742
Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, Johnson DH, Miaskowski C, Scherr SL, Portenoy RK, Vogelzang NJ (2000) Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist 5:353–360
Bayly JL, Lloyd-Williams M (2016) Identifying functional impairment and rehabilitation needs in patients newly diagnosed with inoperable lung cancer: a structured literature review. Support Care Cancer 24:2359–2379
Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, Cleeland C, Dotan E, Eisenberger MA, Escalante CP, Jacobsen PB, Jankowski C, LeBlanc T, Ligibel JA, Loggers ET, Mandrell B, Murphy BA, Palesh O, Pirl WF, Plaxe SC, Riba MB, Rugo HS, Salvador C, Wagner LI, Wagner-Johnston ND, Zachariah FJ, Bergman MA, Smith C (2015) Cancer-related fatigue, version 2. 2015. J Nat Comprehens Cancer Network : JNCCN 13:1012–1039
Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J (2004) Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr:40–50
Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR (2007) Cancer-related fatigue: the scale of the problem. Oncologist 12(Suppl 1):4–10
Eyigor S, Akdeniz S (2014) Is exercise ignored in palliative cancer patients? World J Clin Oncol 5:554–559
Jensen W, Baumann FT, Stein A, Bloch W, Bokemeyer C, de Wit M, Oechsle K (2014) Exercise training in patients with advanced gastrointestinal cancer undergoing palliative chemotherapy: a pilot study. Support Care Cancer 22:1797–1806
Oldervoll LM, Loge JH, Paltiel H, Asp MB, Vidvei U, Wiken AN, Hjermstad MJ, Kaasa S (2006) The effect of a physical exercise program in palliative care: a phase II study. J Pain Symptom Manag 31:421–430
Pyszora A, Budzyński J, Wójcik A, Prokop A, Krajnik M (2017) Physiotherapy programme reduces fatigue in patients with advanced cancer receiving palliative care: randomized controlled trial. Support Care Cancer 25:2899–2908
Poort H, Peters M, van der Graaf WTA, Nieuwkerk PT, van de Wouw AJ, Nijhuis-van der Sanden MWG, Bleijenberg G, Verhagen C, Knoop H (2020) Cognitive behavioral therapy or graded exercise therapy compared with usual care for severe fatigue in patients with advanced cancer during treatment: a randomized controlled trial. Ann Oncol 31:115–122
Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T (2012) Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 62:243–274
Frikkel J, Götte M, Beckmann M, Kasper S, Hense J, Teufel M, Schuler M, Tewes M (2020) Fatigue, barriers to physical activity and predictors for motivation to exercise in advanced cancer patients. BMC Palliat Care 19:43
Granger CL, Connolly B, Denehy L, Hart N, Antippa P, Lin KY, Parry SM (2017) Understanding factors influencing physical activity and exercise in lung cancer: a systematic review. Support Care Cancer 25:983–999
Zopf EM, Newton RU, Taaffe DR, Spry N, Cormie P, Joseph D, Chambers SK, Baumann FT, Bloch W, Galvao DA (2017) Associations between aerobic exercise levels and physical and mental health outcomes in men with bone metastatic prostate cancer: a cross-sectional investigation. Eur J Cancer Care 26
Blaney JM, Lowe-Strong A, Rankin-Watt J, Campbell A, Gracey JH (2013) Cancer survivors’ exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: a questionnaire-survey. Psycho-oncology 22:186–194
Fernandez S, Franklin J, Amlani N, DeMilleVille C, Lawson D, Smith J (2015) Physical activity and cancer: a cross-sectional study on the barriers and facilitators to exercise during cancer treatment. Canadian Oncol Nurs J = Revue canadienne de nursing oncologique 25:37–48
Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Vallance JK, Fairey AS (2005) A longitudinal study of exercise barriers in colorectal cancer survivors participating in a randomized controlled trial. Ann Behav Med 29:147–153
Bohn SH, Fossa SD, Wisloff T, Thorsen L (2019) Physical activity and associations with treatment-induced adverse effects among prostate cancer patients. Support Care Cancer 27:1001–1011
Eng L, Pringle D, Su J, Shen X, Mahler M, Niu C, Charow R, Tiessen K, Lam C, Halytskyy O, Naik H, Hon H, Irwin M, Pat V, Gonos C, Chan C, Villeneuve J, Harland L, Shani RM, Brown MC, Selby P, Howell D, Xu W, Liu G, Alibhai SMH, Jones JM (2018) Patterns, perceptions, and perceived barriers to physical activity in adult cancer survivors. Support Care Cancer 26:3755–3763
Mikkelsen MK, Nielsen DL, Vinther A, Lund CM, Jarden M (2019) Attitudes towards physical activity and exercise in older patients with advanced cancer during oncological treatment - a qualitative interview study. Eur J Oncol Nurs 41:16–23
Blaney J, Lowe-Strong A, Rankin J, Campbell A, Allen J, Gracey J (2010) The cancer rehabilitation journey: barriers to and facilitators of exercise among patients with cancer-related fatigue. Phys Ther 90:1135–1147
Sheill G, Guinan E, Neill LO, Hevey D, Hussey J (2018) The views of patients with metastatic prostate cancer towards physical activity: a qualitative exploration. Support Care Cancer 26:1747–1754
Peters ME, Goedendorp MM, Verhagen CA, Bleijenberg G, van der Graaf WT (2016) Fatigue and its associated psychosocial factors in cancer patients on active palliative treatment measured over time. Support Care Cancer 24:1349–1355
Susanne K, Michael F, Thomas S, Peter E, Andreas H (2019) Predictors of fatigue in cancer patients: a longitudinal study. Support Care Cancer 27:3463–3471
Poort H, de Rooij BH, Uno H, Weng S, Ezendam NPM, van de Poll-Franse L, Wright AA (2020) Patterns and predictors of cancer-related fatigue in ovarian and endometrial cancers: 1-year longitudinal study. Cancer 126:3526–3533
Van Dijk-Lokkart EM, Steur LMH, Braam KI, Veening MA, Huisman J, Takken T, Bierings M, Merks JH, Van den Heuvel-Eibrink MM, Kaspers GJL, Van Dulmen-den Broeder E, Van Litsenburg RRL (2019) Longitudinal development of cancer-related fatigue and physical activity in childhood cancer patients. Pediatric Blood & Cancer 66:e27949
Verkissen MN, Hjermstad MJ, Van Belle S, Kaasa S, Deliens L, Pardon K (2019) Quality of life and symptom intensity over time in people with cancer receiving palliative care: results from the international European Palliative Care Cancer Symptom study. PLoS One 14:e0222988
Stiel S, Matthes ME, Bertram L, Ostgathe C, Elsner F, Radbruch L (2010) Validation of the new version of the minimal documentation system (MIDOS) for patients in palliative care : the German version of the Edmonton symptom assessment scale (ESAS). Schmerz (Berlin, Germany) 24:596–604
Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE (2002) Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manag 24:547–561
Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E (1997) Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manag 13:63–74
Cella D (1997) The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol 34:13–19
Van Belle S, Paridaens R, Evers G, Kerger J, Bron D, Foubert J, Ponnet G, Vander Steichel D, Heremans C, Rosillon D (2005) Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: proposal for use as a screening tool. Support Care Cancer 13:246–254
Kroenke K, Spitzer RL (2002) The PHQ-9: a new depression and diagnostic severity measure. Psychiatr Ann 32:509–521
Kroenke K, Spitzer RL, Williams JB (2001) The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16:606–613
Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH (2009) The PHQ-8 as a measure of current depression in the general population. J Affect Disord 114:163–173
Granger CL, Denehy L, Remedios L, Retica S, Phongpagdi P, Hart N, Parry SM (2016) Barriers to translation of physical activity into the lung cancer model of care. A qualitative study of clinicians’ perspectives. Ann Am Thorac Soc 13:2215–2222
Hefferon K, Murphy H, McLeod J, Mutrie N, Campbell A (2013) Understanding barriers to exercise implementation 5-year post-breast cancer diagnosis: a large-scale qualitative study. Health Educ Res 28:843–856
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Cohen J (1988) Statistical power analysis for the behavioral sciences. L. Erlbaum Associates, Hillsdale, N.J.
Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH (2018) Cancer-associated cachexia. Nat Rev Dis Primers 4:17105
Solheim TS, Laird BJA, Balstad TR, Stene GB, Bye A, Johns N, Pettersen CH, Fallon M, Fayers P, Fearon K, Kaasa S (2017) A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle 8:778–788
Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvão DA (2013) Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis 16:328–335
Tsianakas V, Harris J, Ream E, Van Hemelrijck M, Purushotham A, Mucci L, Green JS, Fewster J, Armes J (2017) CanWalk: a feasibility study with embedded randomised controlled trial pilot of a walking intervention for people with recurrent or metastatic cancer. BMJ Open 7:e013719
Nipp RD, Fuchs G, El-Jawahri A, Mario J, Troschel FM, Greer JA, Gallagher ER, Jackson VA, Kambadakone A, Hong TS, Temel JS, Fintelmann FJ (2018) Sarcopenia Is associated with quality of life and depression in patients with advanced cancer. Oncologist 23:97–104
Beernaert K, Pardon K, Van den Block L, Devroey D, De Laat M, Geboes K, Surmont V, Deliens L, Cohen J (2016) Palliative care needs at different phases in the illness trajectory: a survey study in patients with cancer. Eur J Cancer Care 25:534–543
Dittus KL, Gramling RE, Ades PA (2017) Exercise interventions for individuals with advanced cancer: a systematic review. Prev Med 104:124–132
Ungar N, Wiskemann J, Sieverding M (2016) Physical activity enjoyment and self-efficacy as predictors of cancer patients' physical activity level. Front Psychol 7:898
Ormel HL, van der Schoot GGF, Sluiter WJ, Jalving M, Gietema JA, Walenkamp AME (2018) Predictors of adherence to exercise interventions during and after cancer treatment: a systematic review. Psycho-oncology 27:713–724
Acknowledgements
We would like to acknowledge Adnan Köklü for his helpful language editing.
Availability of data and material
Data is available from the corresponding author on reasonable request.
Code availability
N/A
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MTew generated the objectives; MS and MTew had the project idea; JH, MTeu, and MTew designed the survey; JF and MTew managed administrations; JF, MG, and MTew contributed to literature research; JF, SK, and MTew acquired data; JF, ND, MB, and MTew analyzed and interpreted data and prepared the manuscript; MG, SK, MTeu, MS, and MTew substantively revised it. All authors approved the final manuscript
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and local ethical review committee of the University of Essen and approved the data analysis (17-7385-BO) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was listed at the German Clinical Trials Register (DRKS00012514).
Consent to participate
Consent to participate was obtained from all individual participants included in the study.
Consent for publication
N/A
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frikkel, J., Beckmann, M., De Lazzari, N. et al. Changes in fatigue, barriers, and predictors towards physical activity in advanced cancer patients over a period of 12 months—a comparative study. Support Care Cancer 29, 5127–5137 (2021). https://doi.org/10.1007/s00520-021-06020-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06020-3