Abstract
Background
Lipegfilgrastim has been shown to be non-inferior to pegfilgrastim for reduction of the duration of severe neutropenia (DSN) in breast cancer patients. This open-label, non-inferiority study assessed the efficacy and safety of lipegfilgrastim versus pegfilgrastim in elderly patients with aggressive B cell non-Hodgkin lymphoma (NHL) at high risk for chemotherapy-induced neutropenia.
Patient and methods
One hundred and one patients (median age, 75 years) were randomized to lipegfilgrastim or pegfilgrastim (6 mg/cycle) during six cycles of R-CHOP21.
Results
Lipegfilgrastim was non-inferior to pegfilgrastim for the primary efficacy endpoint, reduction of DSN in cycle 1. In the per-protocol population, mean (standard deviation) DSN was 0.8 (0.92) and 0.9 (1.11) days in the two groups, respectively; the adjusted mean difference between groups was − 0.3 days (95% confidence interval, − 0.70 to 0.19). Non-inferiority was also demonstrated in the intent-to-treat population. The incidence of severe neutropenia in cycle 1 was 51% (21/41) in the lipegfilgrastim group and 52% (23/44) in the pegfilgrastim group. Very severe neutropenia (ANC < 0.1 × 109/L) in cycle 1 was reported by 5 (12%) patients in the lipegfilgrastim group and 8 (18%) patients in the pegfilgrastim group. However, over all cycles, febrile neutropenia (strict definition) was reported by only 1 (2%) patient in each treatment group (during cycle 1 in the lipegfilgrastim group and cycle 6 in the pegfilgrastim group). The mean time to absolute neutrophil count recovery (defined as ≥ 2.0 × 109/L) was 8.3 and 9.4 days in the two groups, respectively. Serious adverse events occurred in 46% of patients in each group; none were considered treatment-related. Eight patients died during the study (2 in the lipegfilgrastim group, 5 in the pegfilgrastim group, and 1 who died before starting study treatment). No deaths occurred during the treatment period, and all were considered to be related to the underlying disease.
Conclusions
This study shows lipegfilgrastim to be non-inferior to pegfilgrastim for the reduction of DSN in elderly patients with aggressive B cell NHL receiving myelosuppressive chemotherapy, with a comparable safety profile.
Trial registration number
ClinicalTrials.gov identifier NCT02044276; EudraCT number 2013-001284-23
Similar content being viewed by others
Introduction
Elderly patients with non-Hodgkin lymphoma (NHL) receiving chemotherapy, such as R-CHOP, are at high risk of developing clinically significant neutropenia [1,2,3], which can lead to dose reductions, cycle delays, or even treatment discontinuation. Clinically significant neutropenia is defined as grade 4 neutropenia (absolute neutrophil count [ANC] < 0.5 × 109/L) according to the Common Terminology Criteria for Adverse Events, the most widely used scale for grading chemotherapy-related cytopenias [4]. Maintenance of chemotherapy intensity is important in patients with NHL, as there is strong evidence that survival is negatively impacted by reductions in relative dose intensity in this population [5,6,7,8]. Use of recombinant granulocyte colony–stimulating factors (G-CSFs) is recommended for patients at high risk of chemotherapy-induced neutropenia [9,10,11], and has been shown to improve survival, especially in elderly patients and those receiving dose-dense regimens [5].
Short-acting G-CSFs, such as filgrastim (Neupogen®; Amgen Inc., Thousand Oaks, CA, USA), require daily subcutaneous injections during each chemotherapy cycle. Pegylation decreases plasma clearance of filgrastim and extends its half-life in the body, allowing for less frequent dosing. Lipegfilgrastim (Lonquex®; Teva B.V., Haarlem, Netherlands) is a long-acting G-CSF indicated for reduction of the duration of neutropenia and the incidence of febrile neutropenia (FN) in adult patients receiving cytotoxic chemotherapy [12]. Lipegfilgrastim is glycopegylated in a site-specific manner, resulting in greater structural homogeneity and improved pharmacokinetic and pharmacodynamic properties compared with conventionally pegylated G-CSFs [13, 14]. Lipegfilgrastim has been shown to induce a longer-lasting increase in ANC than an equivalent dose of the conventionally glycopegalated long-acting G-CSF, pegfilgrastim (Neulasta®; Amgen Inc., Thousand Oaks, CA, USA) [15]. This may reflect the higher cumulative exposure and slower clearance of lipegfilgrastim compared with pegfilgrastim [15]. Lipegfilgrastim, administered once per chemotherapy cycle, has been shown to be non-inferior to pegfilgrastim with respect to duration of severe neutropenia (DSN, defined as the number of days with grade 4 neutropenia [ANC < 0.5 × 109/L]) in breast cancer patients [16].
Pegfilgrastim has been shown to be effective for the reduction of DSN and complications of neutropenia in patients with lymphoma receiving chemotherapy regimens associated with a high risk of FN [17,18,19,20]. A systematic review undertaken to assess the effectiveness of pegfilgrastim in cancer patients in real-world clinical settings found the risks of FN and FN-related complications to be lower in patients receiving pegfilgrastim than in those receiving short-acting G-CSFs (namely, filgrastim, lenograstim, and biosimilars) [21]. In particular, pegfilgrastim has been shown to be effective for the reduction of DSN in elderly patients with NHL receiving myelosuppressive chemotherapy [22]. This study was undertaken to demonstrate non-inferiority of lipegfilgrastim versus pegfilgrastim in elderly patients with aggressive B cell NHL receiving R-CHOP21, and to compare the efficacy and safety of these long-acting G-CSFs in this elderly NHL population.
Methods
Study design and patients
This was a phase 3b, open-label, multicenter study conducted at 31 sites in Germany, Italy, and Spain between March 2014 and December 2017. The study comprised a 2-week screening period, an 18-week, open-label treatment period (6 cycles of R-CHOP21, each of 3 weeks in duration), and a follow-up period of up to 9 months from the start of the first chemotherapy cycle.
Study inclusion and exclusion criteria are summarized in Supplementary Table S1. Patients aged 65–85 years with histologically confirmed aggressive B cell NHL (World Health Organization lymphoma classification criteria [23]) were randomized 1:1 to receive lipegfilgrastim 6 mg or pegfilgrastim 6 mg administered as a single subcutaneous injection on day 3 of each chemotherapy cycle, approximately 24 (± 3) hours after the end of day 2 chemotherapy. During each chemotherapy cycle, patients received (i) rituximab 375 mg/m2 intravenously on day 1; (ii) cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, and vincristine 1.4 mg/m2 (capped at 2.0 or 1.0 mg) intravenously on day 2; and (iii) prednisone or prednisolone 100 mg orally on days 2 to 6.
The study was approved by independent ethics committees at each study site, and complied with the Declaration of Helsinki, Good Clinical Practice guidelines, and applicable local laws and regulations. Patients provided written informed consent to participate.
Study assessments
Blood samples were collected for determination of ANC on days 1, 8, and 15 of each cycle, and on days 3, 5, 10, and 12 of cycle 1. ANC analyses were performed by local laboratories. Patients recorded their oral body temperature daily throughout the study (≤ 1 h before chemotherapy administration on days 1 and 2 and before study drug administration on day 3). Patients were also instructed to measure their body temperature if they felt feverish at any time during the day. If body temperature was > 38.0 °C, patients were instructed to measure their body temperature again after 1 h. Patients were instructed to contact study site personnel if their body temperature was > 38.0 °C for more than 1 h.
The primary efficacy measure was ANC, and the primary efficacy outcome was DSN in cycle 1 (number of days with grade 4 neutropenia [ANC < 0.5 × 109/L]). Secondary efficacy measures included the incidence of FN (body temperature > 38.5 °C for ≥1 h and ANC < 0.5 × 109/L [strict definition], or a single body temperature value ≥ 38.3 °C or body temperature ≥ 38.0 °C for ≥1 h and ANC < 1.0 × 109/L [non-strict definition], including cases of neutropenic sepsis or neutropenic serious or life-threatening infection), the incidence of very severe and severe neutropenia during cycle 1 (ANC < 0.1 × 109/L and < 0.5 × 109/L, respectively), the ANC nadir, and the time to ANC recovery (return to ANC ≥ 1.0, ≥ 1.5, and ≥ 2.0 × 109L) in cycle 1. The incidence and severity of infections, rates of hospitalization and intravenous/oral antibiotic administration, and the percentage of chemotherapy dose delivered were also assessed.
Adverse events (AEs) were monitored throughout the study period (classified and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, v4.03). Quality of life was assessed prior to administration of chemotherapy in cycles 1 and 4 and at the end of treatment using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) [24] and Functional Assessment of Cancer Therapy-Neutropenia (FACT-N) [25].
Statistical analysis
Analysis of the primary endpoint was performed for both the per-protocol and intent-to-treat populations. Efficacy data are shown for the per-protocol population unless otherwise noted. Differences in DSN in cycle 1 between treatment groups were analyzed using the two-sided 95% confidence interval (CI), calculated by Poisson regression with identity link, including treatment, body weight, and country as fixed factors and baseline ANC as a covariate. Lipegfilgrastim was considered non-inferior to pegfilgrastim if the upper limit of the two-sided 95% CI for the difference in DSN between groups (lipegfilgrastim minus pegfilgrastim) was < 1 day. A sample size of 50 patients per treatment group provided at least 85% power to reject the null hypothesis.
Secondary endpoints were analyzed by fitting a logistic regression model including the same explanatory variables, and the 95% CI for the odds ratio (lipegfilgrastim versus pegfilgrastim) was calculated. All secondary endpoint analyses were regarded as exploratory; no adjustment for multiple comparisons was performed.
The safety population included all randomized patients who received at least one dose of study medication. All analyses were performed using SAS statistical software version 9.1 or later (SAS Institute, Inc. Cary, NC, USA).
Results
Study population
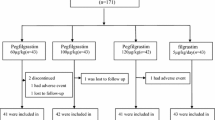
One hundred and one patients (median age, 75 years [range, 65–82 years]) were enrolled and randomized. Patient disposition is summarized in Fig. 1. The two treatment groups were generally well-matched in terms of patient demographics and baseline disease characteristics (Table 1).
Efficacy
Duration of severe neutropenia in cycle 1
Lipegfilgrastim was non-inferior to pegfilgrastim for the reduction in DSN in cycle 1 (Table 2). Mean (standard deviation [SD]) DSN in cycle 1 was 0.8 (0.92) and 0.9 (1.11) days in the two groups, respectively. The adjusted mean DSN difference between groups was − 0.3 days (95% CI, − 0.70 to 0.19). Non-inferiority was also demonstrated in the intent-to-treat population (adjusted mean DSN difference between groups, − 0.1 days [95% CI, − 0.56 to 0.30]).
Incidence of febrile neutropenia and severe neutropenia
Over all cycles, FN according to the strict definition was reported in 1 (2%) patient in each treatment group (during cycle 1 in the lipegfilgrastim group and cycle 6 in the pegfilgrastim group). When assessed using the non-strict definition, FN was reported by 5 (12%) and 2 (5%) patients, respectively.
Very severe neutropenia (ANC < 0.1 × 109/L) in cycle 1 was reported by 5 (12%) patients in the lipegfilgrastim group and 8 (18%) patients in the pegfilgrastim group (adjusted odds ratio, 0.51 [95% CI, 0.131 to 2.016]). Severe neutropenia (ANC < 0.5 × 109/L) was reported by 21 (51%) and 23 (52%) patients, respectively (adjusted odds ratio, 0.99 [95% CI, 0.396 to 2.470]).
Absolute neutrophil counts
Mean daily ANC during cycle 1 is shown in Supplementary Table S2; mean ANC across all cycles is shown in Fig. 2. In both groups, the highest ANC was observed on day 5 of cycle 1. During subsequent cycles, mean ANC peaked on day 15, and was consistently higher in the lipegfilgrastim group. In cycle 1, the ANC nadir was reached around day 10 and was similar in both groups (1.00 ± 1.36 × 109L and 1.19 ± 1.92 × 109L, respectively). The mean time to ANC recovery (≥ 2.0 × 109/L) was 8.3 ± 3.30 days in the lipegfilgrastim group and 9.4 ± 4.92 days in the pegfilgrastim group (Table 3).
Incidence and severity of infection
Over all cycles, infection was reported in 16 (39%) patients in the lipegfilgrastim group and 6 (14%) patients in the pegfilgrastim group (adjusted odds ratio, 4.61 [95% CI, 1.43 to 14.89]). Most infections occurred during cycle 1 (6/16 and 4/6 patients in the two groups, respectively). There was only one infection of grade 4 severity during the study (sepsis in the pegfilgrastim group). Infections of any severity reported by more than a single patient in either group were viral upper respiratory tract infection, herpes zoster, bronchitis, conjunctivitis, oral candidiasis, urinary tract infection, infection, cystitis, influenza, and fungal infection. Infection was microbiologically documented in 4/16 and 2/6 patients in the two groups, respectively.
Incidence of hospitalization and antibiotic administration due to febrile neutropenia
Hospitalization due to FN was reported in 5 (12%) patients in the lipegfilgrastim group and 1 (2%) patient in the pegfilgrastim group (all during cycle 1). Intravenous or oral antibiotics were prescribed as treatment or prophylaxis for FN in 16 (39%) and 4 (9%) patients in the two groups, respectively.
Chemotherapy dose and delivery
Across all cycles, the median cumulative percentage of the scheduled chemotherapy dose actually delivered was 100% for all drugs in both groups, except for vincristine in the pegfilgrastim group (71.4%). Chemotherapy was administered as planned to all patients in cycle 1. Over cycles 2 to 6, 32 (80%) patients in the lipegfilgrastim group and 33 (75%) in the pegfilgrastim group had delays in their chemotherapy treatment. Two (5%) patients in the pegfilgrastim group omitted at least one chemotherapy cycle. The overall incidence of chemotherapy dose reduction was low in both groups.
Safety
Almost all patients (98%) reported at least one AE (Supplementary Table S3). The only AE occurring more frequently (≥ 10% difference between groups) in patients receiving lipegfilgrastim was cough. AEs occurring more frequently in patients receiving pegfilgrastim were anemia, nausea, diarrhea, and weight decrease. Bone pain was reported in 2 (4%) patients in the lipegfilgrastim group and 3 (6%) in the pegfilgrastim group. AEs were considered at least possibly related to treatment in 11 (24%) and 10 (20%) patients in the two groups, respectively. No treatment-related AE was reported by more than two patients in either group.
Serious AEs occurred in 46% of patients (21/46 in the lipegfilgrastim group and 23/50 in the pegfilgrastim group). Ten patients withdrew from the study due to AEs (1 [2%] in the lipegfilgrastim group and 9 [18%] in the pegfilgrastim group), none of which were considered treatment-related. Eight patients died during the study (2 in the lipegfilgrastim group, 5 in the pegfilgrastim group, and 1 patient randomized to lipegfilgrastim who died before starting study treatment). No deaths occurred during the treatment period, all were considered to be related to the underlying disease, and none were due to infection.
Quality of life
There were no noteworthy differences between groups or changes over time for any of the EORTC QLQ-C30 or FACT-N scores over the study period.
Discussion
This study demonstrated non-inferiority of lipegfilgrastim compared with pegfilgrastim for the reduction of DSN in elderly patients with aggressive B cell NHL receiving myelosuppressive chemotherapy. Results of the analysis in the intent-to-treat population were similar to those in the per-protocol population, confirming the robustness of this finding. Lipegfilgrastim also demonstrated generally comparable efficacy to pegfilgrastim across all secondary endpoints. The incidence of FN was low in both treatment groups, and there were no clinically relevant differences in the incidence of severe or very severe neutropenia, incidence of DSN by duration, depth and time of the ANC nadir, delays in chemotherapy administration, or any quality-of-life measures. Of note, the median cumulative percentage of the scheduled chemotherapy dose actually delivered was 100% for all drugs in both groups, with the exception of vincristine in the pegfilgrastim group. To date, few other studies have assessed the clinical utility of long-acting G-CSFs in this specific patient population [22].
The risk of developing FN in patients receiving R-CHOP21 without G-CSF prophylaxis according to the strict definition used in this study is estimated to be 10–20% [2, 3, 9]. The corresponding incidence of FN in this study was 2% in both groups. This represents a reduction in the incidence of FN of approximately 90%, highlighting the value of treatment with lipegfilgrastim or pegfilgrastim in this patient population. During the study, six patients were hospitalized due to investigator-defined FN (5 in the lipegfilgrastim group and 1 in the pegfilgrastim group). These hospitalizations did not result in an increase in morbidity and mortality, and it could be that other safety issues influenced the need for hospital admission in this high-risk population of elderly cancer patients. The incidence of infection was somewhat higher in the lipegfilgrastim group than in the pegfilgrastim group, but this did not appear to correlate with low neutrophil counts, compromise chemotherapy treatment or lead to increased AEs or serious AEs. Infections were predominantly of grade 3 severity or lower, and the only grade 4 infection occurred in the pegfilgrastim group.
The safety profile of lipegfilgrastim was similar to that of pegfilgrastim, and no new safety signals for lipegfilgrastim were identified. The incidence of AEs was as expected in a population of elderly patients with NHL receiving myelosuppressive chemotherapy, and reported AEs were consistent with the underlying disease and the chemotherapy regimen administered. Bone pain was reported by few patients. More patients withdrew from the study due to AEs in the pegfilgrastim group than in the lipegfilgrastim group; however, none of the AEs leading to withdrawal were considered treatment-related. None of the 8 deaths during the study occurred during the active treatment phase, and all were considered to be related to the underlying disease.
Results of this study are in line with those of a subanalysis of the prospective, non-interventional NADIR study undertaken to evaluate the effectiveness and safety of lipegfilgrastim in patients with NHL undergoing chemotherapy in routine practice settings [26]. A meta-analysis and indirect treatment comparison of lipegfilgrastim versus pegfilgrastim and filgrastim for the reduction of chemotherapy-induced neutropenia and related events demonstrated significant and clinically meaningful differences in favor of lipegfilgrastim for both time to ANC recovery (which is typically longer than DSN) and risk of severe neutropenia [27]. Lipegfilgrastim was also associated with a lower risk of FN over all cycles; however, differences between groups were not statistically significant [27]. As a result of its novel pegylation method [13, 14], lipegfilgrastim has a different pharmacokinetic and pharmacodynamic profile than pegfilgrastim, specifically higher cumulative exposure and slower clearance, and induces a longer-lasting increase in ANC at equivalent doses [15]. Analyses utilizing data from patients with breast cancer suggest that lipegfilgrastim is likely to be a cost-effective alternative to pegfilgrastim for primary prohylaxis of complications of chemotherapy-induced neutropenia and associated complications [28, 29].
In conclusion, this study shows that lipegfilgrastim is an effective option to reduce the duration of severe chemotherapy-induced neutropenia and to prevent febrile neutropenia in elderly patients with aggressive B cell NHL receiving myelosuppressive chemotherapy.
Data availability
The authors confirm to have full control of all primary data.
Qualified researchers may request access to patient level data and related study documents including the study protocol and the statistical analysis plan. Requests will be reviewed for scientific merit, product approval status, and conflicts of interest. Patient level data will be de-identified and study documents will be redacted to protect the privacy of trial participants and to protect commercially confidential information. Please email USMedInfo@tevapharm.com to make your request.
References
Coiffier B, Lepage E, Briere J et al (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346(4):235–242
Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, Pocock C, Ardeshna KM, Radford JA, McMillan A, Davies J, Turner D, Kruger A, Johnson P, Gambell J, Linch D (2013) Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet 381(9880):1817–1826
Delarue R, Tilly H, Mounier N, Petrella T, Salles G, Thieblemont C, Bologna S, Ghesquières H, Hacini M, Fruchart C, Ysebaert L, Fermé C, Casasnovas O, van Hoof A, Thyss A, Delmer A, Fitoussi O, Molina TJ, Haioun C, Bosly A (2013) Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol 14(6):525–533
National Cancer Institutes. Common Terminology Criteria for Adverse Advents v4.03 (Excel). June 14, 2010. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. Accessed 29 June 2020
Lyman GH, Dale DC, Culakova E, Poniewierski MS, Wolff DA, Kuderer NM, Huang M, Crawford J (2013) The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol 24(10):2475–2484
Kwak LW, Halpern J, Olshen RA, Horning SJ (1990) Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: results of a tree-structured survival analysis. J Clin Oncol 8(6):963–977
Bosly A, Bron D, Van Hoof A et al (2008) Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol 87(4):277–283
Pettengell R, Schwenkglenks M, Bosly A (2008) Association of reduced relative dose intensity and survival in lymphoma patients receiving CHOP-21 chemotherapy. Ann Hematol 87(5):429–430
Aapro MS, Bohlius J, Cameron DA, Lago LD, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47(1):8–32
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, Goldberg JM, Khatcheressian JL, Leighl NB, Perkins CL, Somlo G, Wade JL, Wozniak AJ, Armitage JO (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 33(28):3199–3212
Klastersky J, de Naurois J, Rolston K, Rapoport B, Maschmeyer G, Aapro M, Herrstedt J, ESMO Guidelines Committee (2016) Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol 27:v111–v118
European Medicines Agency. Lonquex 6 mg solution for injection in pre-filled syringe. Summary of product characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/lonquex-epar-product-information_en.pdf. Accessed 29 June 2020.
Mahlert F, Schmidt K, Allgaier H, Liu P, Müller U, Shen WD (2013) Rational development of lipegfilgrastim, a novel long-acting granulocyte colony-stimulating factor, using glycopegylation technology. Blood 122(21):4853
Scheckermann C, Schmidt K, Abdolzade-Bavil A, Allgaier H, Mueller UW, Shen WD, Liu P (2013) Lipegfilgrastim: a long-acting, once-per-cycle, glycopegylated recombinant human filgrastim. J Clin Oncol 31:e13548
Buchner A, Lammerich A, Abdolzade-Bavil A, Müller U, Bias P (2014) Lipegfilgrastim: pharmacodynamics and pharmacokinetics for body-weight-adjusted and 6 mg fixed doses in two randomized studies in healthy volunteers. Curr Med Res Opin 30(12):2523–2533
Bondarenko I, Gladkov OA, Elsaesser R, Buchner A, Bias P (2013) Efficacy and safety of lipegfilgrastim versus pegfilgrastim: a randomized, multicenter, active-control phase 3 trial in patients with breast cancer receiving doxorubicin/docetaxel chemotherapy. BMC Cancer 13:386–398
Pettengell R, Schwenkglenks M, Bacon P, Lawrinson S, Duehrsen U (2011) Pegfilgrastim primary prophylaxis in patients with non-Hodgkin lymphoma: results from an integrated analysis. Hematol Oncol 29(4):177–184
Kubo K, Miyazaki Y, Murayama T, Shimazaki R, Usui N, Urabe A, Hotta T, Tamura K (2016) A randomized, double-blind trial of pegfilgrastim versus filgrastim for the management of neutropenia during CHASE(R) chemotherapy for malignant lymphoma. Br J Haematol 174(4):563–570
Cerchione C, De Renzo A, Di Perna M et al (2017) Pegfilgrastim in primary prophylaxis of febrile neutropenia following frontline bendamustine plus rituximab treatment in patients with indolent non-Hodgkin lymphoma: a single center, real-life experience. Support Care Cancer 25(3):839–845
Salmon JP, Smakal M, Karanikiotis C, Wojtukiewicz MZ, Omnes Y, DeCosta L, Wetten S, O’Kelly J (2019) Febrile neutropenia (FN) and pegfilgrastim prophylaxis in breast cancer and non-Hodgkin’s lymphoma patients receiving high (> 20%) FN-risk chemotherapy: results from a prospective observational study. Support Care Cancer 27(4):1449–1457
Mitchell S, Li X, Woods M, Garcia J, Hebard-Massey K, Barron R, Samuel M (2016) Comparative effectiveness of granulocyte colony-stimulating factors to prevent febrile neutropenia and related complications in cancer patients in clinical practice: a systematic review. J Oncol Pharm Pract 22(5):702–716
Grigg A, Solal-Celigny P, Hoskin P, Taylor K, M A (2003) Open-label, randomized study of pegfilgrastim vs. daily filgrastim as an adjunct to chemotherapy in elderly patients with non-Hodgkin’s lymphoma. Leuk Lymphoma 44(9):1503–1508
Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES (2011) The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 117(19):5019–5032
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, Haes JCJM, Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–376
Wagner LI, Beaumont JL, Ding B, Malin J, Peterman A, Calhoun E, Cella D (2008) Measuring health-related quality of life and neutropenia-specific concerns among older adults undergoing chemotherapy: validation of the Functional Assessment of Cancer Therapy-Neutropenia (FACT-N). Support Care Cancer 16(1):47–56
Wolff T, Schulz H, Losem C, Reichert D, Hurtz HJ, Sandner R, Harde J, Grebhardt S, Potthoff K, Mueller U, Fietz T (2019) Prophylaxis of chemotherapy-induced neutropenia and febrile neutropenia with lipegfilgrastim in patients with non-Hodgkin lymphoma (NADIR study). Eur J Haematol 102(2):174–181
Bond TC, Szabo E, Gabriel S, Klastersky J, Tomey O, Mueller U, Schwartzberg L, Tang B (2018) Meta-analysis and indirect treatment comparison of lipegfilgrastim with pegfilgrastim and filgrastim for the reduction of chemotherapy-induced neutropenia-related events. J Oncol Pharm Pract 24(6):412–423
Akpo EIH, Jansen IR, Maes E, Simoens S (2017) Cost-utility analysis of lipegfilgrastim compared to pegfilgrastim for the prophylaxis of chemotherapy-induced neutropenia in patients with stage ii-iv breast cancer. Front Pharmacol 8:614
Gao L, Li SC (2018) Cost-effectiveness analysis of lipegfilgrastim as primary prophylaxis in women with breast cancer in Australia: a modelled economic evaluation. Breast Cancer 25(6):671–680
Acknowledgments
Editorial support was provided by Jennifer Coward and Uta Gomes, and was funded by TEVA Europe B.V.
Funding
The AVOID study and preparation of this manuscript were supported by TEVA Europe B.V.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Confict of interest
HL declares to have received personal fees from Teva, Amgen, Chugai, Hexal-Sandoz-Novartis, Mundipharma, Accord Healthcare, and G1 Therapeutics; AK has received a grant from Amgen; AS has received grants from Roche and Gilead and personal fees from Roche, Gilead, Celgene, and Janssen; MZ has received personal fees from Novartis, Hexal, Pfizer, Roche; GI, ME, RD, SM, and UM have no disclosures to declare; PB, AB, and AL are employees of Teva and hold stock options in the company.
Additional information
Key message
Lipegfilgrastim is non-inferior to pegfilgrastim for the reduction of duration of severe neutropenia in elderly patients with aggressive B-NHL receiving myelosuppressive chemotherapy (R-CHOP21), with a comparable safety profile.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 237 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Link, H., Illerhaus, G., Martens, U.M. et al. Efficacy and safety of lipegfilgrastim versus pegfilgrastim in elderly patients with aggressive B cell non-Hodgkin lymphoma (B-NHL): results of the randomized, open-label, non-inferiority AVOID neutropenia study. Support Care Cancer 29, 2519–2527 (2021). https://doi.org/10.1007/s00520-020-05711-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05711-7