Abstract
Purpose
Cancer is of increasing prevalence in less-developed countries. However, research on the patients’ quality of life (QoL) in these countries is very limited. The aim of this study was to examine QoL of cancer patients in Africa.
Method
A sample of 256 cancer patients treated in an Ethiopian hospital was examined with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire EORTC QLQ-C30, the Multidimensional Fatigue Inventory, and the Hospital Anxiety and Depression Scale. A group of 1664 German cancer patients served as a comparison group.
Results
Most of the scales of the EORTC QLQ-C30 showed acceptable reliability in the Ethiopian sample. Compared with the German cancer patients, the Ethiopian patients showed lower QoL in most dimensions, especially in financial difficulties, physical functioning, pain, and appetite loss (effect sizes between 0.52 and 0.75). Illiteracy, tumor stage, and treatment (surgery and chemotherapy) were associated with QoL in the Ethiopian sample. QoL was strongly correlated with fatigue, anxiety, and depression.
Conclusion
The EORTC QLQ-C30 is a suitable instrument for measuring QoL in Ethiopia. The detriments in QoL in the Ethiopian patients indicate specific cancer care needs for the patients in a developing country.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, the incidence of cancer was estimated to be 18.1 million, and the estimation for cancer mortality was 9.5 million in 2018 [1]. Cancer is the first or second leading cause of death before the age 70 in 91 of 172 countries [2]. In Africa, the incidence and mortality rates are lower than the worldwide average. Though the African proportion of the world population is 16.8%, the shares of cancer incidence and cancer mortality are 5.8% and 7.3%, respectively [2]. With increasing life expectancy, however, the cancer incidence and mortality rates are increasing in Africa [3,4,5,6], but it is difficult to obtain reliable epidemiological data in this region.
Quality of life (QoL) has gained increasing relevance in oncological research and treatment in the last decades [7, 8]. One of the most frequently used questionnaires for measuring QoL in cancer patients is the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire EORTC QLQ-C30 [9]. It has been translated into more than 80 languages, and a large number of studies have used this questionnaire in samples of cancer patients, patients suffering from other diseases, and also in the general population. Normative values are available for several countries [10,11,12,13]. Most of the studies on QoL in cancer patients have been performed in Western countries.
In less-developed countries, psycho-oncological care and research is limited. Ethiopia ranks 173rd of the 183 countries in the Human Development Index [14]. Several studies have already been performed using the EORTC QLQ-C30 in Ethiopia [15,16,17,18,19]. All these five studies (with one exception) were performed at Tikur Anbessa Referral Hospital in Addis Ababa, the only oncology referral and radiotherapy center of the whole country. These previous studies in Ethiopia did not triangulate the EORTC QLQ-C30 with data of other symptom and psychological distress measures. Therefore, it is relevant to study the QoL of cancer patients treated outside the capital using the EORTC QLQ-C30 and related measures to contribute to the evaluation of the validity of the EORTC QLQ-C30.
To better evaluate the QoL of the Ethiopian patients, we also compared it with that obtained in a large German study to which we had access to the original data. This enabled us to select a sub-sample so the age and gender distributions of both samples were equivalent.
One common problem observed among the Ethiopian studies on cancer patients so far is that many patients are illiterate. In these cases, the study assistants have to read the questions aloud, ask the patients to respond verbally, and mark the response in the questionnaire. It has not been systematically studied whether such a procedure of data collection has a substantial impact on the outcome. Therefore, we analyzed the difference between illiterate and literate patients in responding to the measures we used.
A better understanding of the level of QoL of cancer patients and of factors influencing QoL can lead to raising awareness, promoting the development of policies in cancer care and facilitating better targeted use of limited resources in less-developed countries.
The specific objectives of this study were (a) to determine the level of QoL in Ethiopian cancer patients in comparison with German cancer patients, (b) to test psychometric properties of the questionnaire EORTC QLQ-C30 applied to Ethiopian cancer patients, (c) to analyze the impact of socio-demographic and clinical variables on QoL, and (d) to examine the correlations between the facets of QoL and several other scales.
Methods
Ethiopian cancer patients
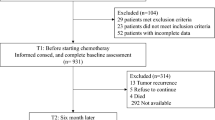
This study was performed at the University of Gondar Hospital. Gondar is a town in Northwestern Ethiopia with about 300,000 inhabitants. Inclusion criteria for the study were a cancer diagnosis, age 18 and above, and the ability to understand (not to read) the Amharic language. A total of 298 cancer patients were eligible for this study performed between January 2019 and June 2019, of which 256 completed the questionnaire. There were no exclusion criteria concerning tumor entities, disease stage, and illiteracy. Trained research assistants contacted the patients, explained the aims of the study, and asked them to participate and to give informed consent. If the patients were illiterate, the research assistants read the questions aloud, asked the patients to respond verbally, and then marked the response in the questionnaire. The study comprised several questionnaires. Medical data was taken from the medical records of the hospital. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the University of Gondar (Ref. No. O/V/P/RCS/05/1542/2018; dated June 18, 2018).
German cancer patients
The sample of the German cancer patients was taken from a large psycho-oncological study performed in five study centers in Germany. Detailed information on the sample and the study methods is available in [20]. The ethics committees of all participating study centers approved the study. Data on QoL have already been published [21]. The sample consisted of 1952 males and 2068 females; the mean age was 58.4 years. Due to the differences in the age and gender distribution between the Ethiopian and German sample, we selected a sub-sample of the German cancer patients so the age and gender distributions matched those of the sample from Ethiopia. This procedure resulted in 1664 German cancer patients, 638 males (38.3%), and 1026 females (61.7%). The mean age of this group was 48.0 years, very similar to that of the Ethiopian sample.
Instead of the German sample, we could also have used the mean scores of two broader pooled samples of cancer patients, either 23,553 cancer patients whose mean scores are reported in the EORTC QLQ-C30 reference values manual [22], or 6024 cancer patients included in a pooled analysis [23]. The advantages of these two pooled samples are higher sample sizes with origins from several countries. The disadvantages, however, are that the samples were taken from randomized trials (which means a certain selection bias) and that we had no access to the original data, so we could not select an age- and gender-matched sub-sample. Additionally, the study with the 6024 patients included a high proportion of melanoma patients. For these reasons, we used the German sample as comparison group.
Instruments
The EORTC QLQ-C30 [9] consists of 30 items assigned to five functioning scales (physical, role, emotional, social, and cognitive functioning), three symptom scales (fatigue, pain, and nausea/vomiting), a two-item global health/QoL scale, and six single items (dyspnea, appetite loss, insomnia, constipation, diarrhea, and financial difficulties). Higher functioning scores indicate better functioning/QoL, whereas higher symptom scores represent more severe symptoms. According to a recommendation of the EORTC Quality of Life Group [24], a sum score of the EORTC QLQ-C30 can be calculated, integrating five functioning scales and eight symptom scales.
Fatigue was measured with the Multidimensional Fatigue Inventory (MFI-20) [25]. This 20-item questionnaire assesses five dimensions of fatigue: general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. In our study, we used the sum score of all the 20 items. Anxiety and depression were measured with the Hospital Anxiety and Depression Scale (HADS) [26]. This questionnaire consists of 14 items. In this study, we used the HADS total score, integrating across all 14 items [27].
Statistical analysis
Mean score differences were expressed in terms of Cohen’s effect sizes d. Reliability was measured with Cronbach’s α coefficient. Two-factor ANOVAs were calculated to test the impact of gender and age group (two categories) on QoL. The impact of further socio-demographic and clinical variables was tested with three-factor ANOVAs with gender and age group as co-variates. Associations between continuous variables were calculated as Pearson correlations. All statistical analyses were performed with SPSS version 24.
Results
Socio-demographic characteristics of the sample
In total, 298 patients were eligible for the study. Of these, 256 (86%) were willing to give informed consent and complete the questionnaires. The research assistants checked the questionnaires for missing items which were then completed. The mean age of the sample was 47.9 years (SD = 14.6 years). Further socio-demographic and clinical variables are given in Table 1. A sub-group of 135 patients was illiterate.
EORTC QLQ-C30 mean scores: comparison between Ethiopian and German cancer patients
The mean scores of the scales are given in Table 2, separately for the Ethiopian and the German sample. The Ethiopian patients reported worse QoL than the German patients; the sum scores yielded an effect size of d = − 0.26. Among the scales of the questionnaires, the highest differences in terms of effect sizes were found for financial difficulties (d = 0.75) and physical functioning (d = − 0.64). The reliability coefficients α of the scales were between 0.64 and 0.90 in the Ethiopian sample with the exception of the cognitive functioning scale.
Age and gender differences in QoL
Figure 1 illustrates the mean scores of the QoL sum score, broken down by age group and gender. The lowest QoL was found for the Ethiopian young male patients. The ANOVA results of the Ethiopian patients were age group, F = 1.32, p = 0.251; gender, F = 3.14, p = 0.077; and age group * gender, F = 4.54, p = 0.034. The impact of age and gender on the single dimensions of QoL is shown in the following section.
The impact of socio-demographic and clinical variables on QoL in the Ethiopian sample
Tables 3 and 4 present the impact of socio-demographic and clinical variables on QoL. Table 3 contains the results for the functioning scales, the global health/QoL scale, while Table 4 contains the results for the symptom scales and the sum score.
Males reported lower levels of role and social functioning and more financial difficulties than females. Concerning age, there was only one significant difference: older patients reported better emotional functioning than younger patients. Illiterate patients showed worse QoL than literate patients in all of the 15 dimensions; the differences were statistically significant in three of the dimensions. Concerning tumor type, there was only one significant difference in the fatigue level. Tumor stage was strongly associated with multiple dimensions of QoL. While the mean scores for stages 1 to 3 did not show large differences, patients with stage 4 reported lowest functioning scores and highest levels of symptoms in most of the scales. Patients receiving surgery or chemotherapy were characterized by better QoL than those patients not receiving those treatments.
Correlations between QoL scales and other scales
Correlations within the Ethiopian sample between the scales of the EORTC QLQ-C30 and the two-item global health/QoL scale, the MFI-20 sum score, and the sum score of the HADS are presented in Table 5. The global assessment of health/QoL was most strongly associated with pain (r = − 0.59) and fatigue (r = − 0.58). The sum score of the fatigue questionnaire MFI-20 correlated most highly with the fatigue scale of the EORTC QLQ-C30 (r = 0.74), and anxiety and depression (HADS total scale) were most strongly associated with the EORTC QLQ-C30 scales emotional functioning, fatigue, and pain (r = 0.65 each).
Discussion
Most of the EORTC QLQ-C30 scales proved to have sufficient reliability. Due to the small number of items per scale, it is not surprising that the EORTC QLQ-C30 scales did not reach the levels of reliability achieved by other questionnaires with more items per scale. The reliability of the cognitive functioning scale (α = 0.46) was very low; however, another Ethiopian study [16] and a study from Tanzania [28] reported even lower coefficients, both below 0.40. The relatively low alpha coefficients for cognitive functioning, social functioning, and nausea/vomiting indicate there is some insecurity in the measurement on the individual level; however, on a group level, assessments are justified.
The comparison between the Ethiopian and the German cancer patients shows the burden is higher in the Ethiopian sample in most of the scales. All functioning scales (except emotional functioning) showed lower mean scores in the Ethiopian sample and all symptom scales (except insomnia and diarrhea) showed higher scores for the patients in Ethiopia. The most pronounced differences were found for financial difficulties (d = 0.74). Two other studies with Ethiopian cancer patients [17, 29] also reported high mean scores in these scales (64.8 and 69.9, respectively), similar to the mean of our Ethiopian study (67.1) and markedly higher than the mean score obtained in Germany (37.8). This difference illustrates that due to the lack of a health insurance system, the financial burden of cancer patients is considerable and this non-clinical factor is relevant for the QoL of patients in developing countries. In the Tanzanian study, the mean score of financial difficulties (84.3) was even higher than that in Ethiopia. Appetite loss and pain were also more pronounced in the Ethiopian sample when compared with the German one with effect sizes of d = 0.60 and d = 0.54, respectively. This is possibly due to the lower availability of adequate medication and lack of pain management interventions in Ethiopia.
However, the meaningfulness of the comparisons between German and Ethiopian cancer patients is limited by the fact that no Ethiopian normative values exist. Thus, we cannot conclude which part of the differences is caused by different medical care conditions and which part is caused by different response behavior in the general population in these countries. Normative studies from developing countries would help clarify this problem.
If we had considered the data of the EORTC QLQ-C30 reference values manual [22] instead of the German data, the differences to the Ethiopian mean scores would have been even larger. With the exception of the constipation scale, the manual reported better QoL than that of the German cancer patients’ sample, making the difference to the Ethiopian sample even greater. For the physical functioning scale (M = 76.7 in the manual), e.g., the effect size increases from d = − 0.64 (German vs. Ethiopian sample) to d = − 0.86 (manual vs. Ethiopian sample). However, the participants of the samples considered in the manual are of other age and gender distribution than the Ethiopian sample, and being included in a clinical trial (patients of the manual) might also produce a certain selection bias since patients with severe diseases are less likely to be included in clinical trials.
It is interesting to note that despite the differences between the Ethiopian and the German sample in the sum score and in several of the functioning and symptom scales, the assessments of the two-item global health/quality of life scale were nearly equal in both samples, M = 54.6 in Ethiopia and M = 54.4 in Germany. A study with comparisons between cancer patients and the general population showed the patients reported severe detriments in several dimensions of QoL when compared with the general population, but they nevertheless rated their global QoL as relatively good [30]. A global assessment of one’s health/QoL is therefore not identical with the sum of the assessments of specific symptoms and functioning facets. One implication of this fact is that examinations of QoL in cancer patients should not be restricted to such a global assessment of health or QoL since the differences found in this global scale can underestimate the real differences.
Illiterate patients reported lower QoL mean sum scores than those who could read and write. However, this group difference was not statistically significant. The differences between the cancer types also failed to reach the significance level. However, we observed a clear and nearly linear association between tumor stage and QoL (p < 0.001). Patients with tumor stage 4 reported the most severe detriments, especially in the domains physical functioning and fatigue. While this relationship is easily understandable and in line with other studies [29, 31], it is interesting to note that patients receiving surgery or chemotherapy reported better QoL than those patients not receiving that treatment. This relationship cannot be attributed to group differences in the distribution of the tumor stages: the proportion of patients receiving chemotherapy varied between 58 and 62% in the four stage groups, and concerning surgery there was a (non-linear) association between receiving surgery and tumor stage with the highest surgery proportions for stage 2 (64%) and stage 3 (60%), while the proportions were lower for stage 1 (55%) and stage 4 (39%). The result of better QoL in patients receiving certain treatment was not found in previous studies from the middle-income countries Malaysia [32] and Taiwan [33]. These differences could be explained partly by the low level of economic standards of the Ethiopian cancer patients who might not be able to get the necessary treatment when needed, which could have contributed to their higher level of symptoms. On the other hand, receiving surgery or chemotherapy might have given the Ethiopian cancer patients the feeling of getting relief from such symptoms. Further research is needed to explain this effect.
The correlations between the specific EORTC QLQ-C30 scales and global health/QoL scale (Table 5) show that all specific aspects of QoL contributed to the global assessment of the patients’ health/QoL, whereby pain (r = − 0.59) and fatigue (r = 0.58) had the most relevant impact. A cross-cultural study [34] analyzed the relationship between the specific QoL scales and the global health/QoL scale in 10 different regions of the world. In seven of these 10 regions, fatigue correlated most highly with global health/QoL, while emotional functioning and pain were the next relevant dimensions. Unfortunately, Africa was not included in this analysis.
The high correlation between the MFI-20 fatigue total score and the EORTC QLQ-C30 fatigue scale (r = 0.74) indicates convergent validity of the fatigue scale. The correlation between the HADS total score (anxiety and depression) and the EORTC QLQ-C30 scales was highest for the scales emotional functioning (r = − 0.65), fatigue (r = 0.65), pain (r = 0.65), and cognitive functioning (r = − 0.64). Together with the high correlation between fatigue and global health/QoL, the results show that fatigue (an overwhelming feeling of exhaustion and tiredness) is a severe problem which strongly impairs mental well-being and QoL. The crucial role of cancer-related fatigue has been documented in multiple studies [35]. Physicians who sometimes tend to overlook this symptom should be aware of its relevance.
This study has several limitations. It was performed in one hospital in Gondar, Ethiopia, and hence, the generalizability to other clinics or countries remains open. About half the patients could not read the questionnaire. The impact of reading the questions aloud by the study assistants on the response behavior has not been systematically analyzed. Though other studies performed in developing countries also used this method, there is no systematic comparison of the psychometric properties of the questionnaires between illiterate and literate patients. Further research should clarify whether there are such systematic differences, irrespective of the specific content of the questionnaires. Several clinical factors were not independent of each other; therefore, the impact of these factors on the QoL assessments may partly be mediated by other factors, even if the significance tests of Tables 3 and 4 considered the potential confounders age and gender. The German sample of cancer patients was designed to be roughly representative of all cancer patients in Germany; however, the data cannot be generalized to “Western countries.” Even within Europe, there are differences in the EORTC QLQ-C30 mean scores of the general population, the means of the global health/QoL scale range from 60.0 in Poland to 77.4 in the Netherlands [10].
In summary, the EORTC QLQ-C30 proved to be fairly applicable to Ethiopian cancer patients speaking the Amharic language, even when patients were illiterate. The differences between the Ethiopian and the German mean scores (financial difficulties, physical functioning, pain, fatigue, appetite loss) show specific cancer care needs for the population in a developing country.
References
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F (2019) Estimating the global cancer incidence and mortality in 2018. Int J Cancer 144(8):1941–1953. https://doi.org/10.1002/ijc.31937
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Woldeamanuel YW, Girma B, Teklu AM (2013) Cancer in Ethiopia. Lancet Oncol 14(4):289–290. https://doi.org/10.1016/S1470-2045(12)70399-6
Hemminki K, Miller AB (2015) Special section editorial. Int J Cancer 137(9):2043–2044. https://doi.org/10.1002/ijc.29696
Adeloye D, Sowunmi OY, Jacobs W, David RA, Adeosun AA, Amuta AO, Misra S, Gadanya M, Auta A, Harhay MO, Chan KY (2018) Estimating the incidence of breast cancer in Africa. J Glob Health 8(1):10419. https://doi.org/10.7189/jogh.08.010419
Parkin DM, Sitas F, Chirenje M, Stein L, Abratt R, Wabinga H (2008) Part I. Lancet Oncol 9(7):683–692. https://doi.org/10.1016/S1470-2045(08)70175-X
Osoba D (1999) What has been learned from measuring health-related quality of life in clinical oncology. Eur J Cancer 35(11):1565–1570
Bottomley A, Flechtner H, Efficace F, Vanvoorden V, Coens C, Therasse P, Velikova G, Blazeby J, Greimel E (2005) Health related quality of life outcomes in cancer clinical trials. Eur J Cancer 41(12):1697–1709. https://doi.org/10.1016/j.ejca.2005.05.007
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, Dehaes JCJM, Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F (1993) The European-Organization-For-Research-And-Treatment-Of-Cancer QLQ-C30 - a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–376. https://doi.org/10.1093/jnci/85.5.365
Nolte S, Liegl G, Petersen MA, Aaronson NK, Costantini A, Fayers PM, Groenvold M, Holzner B, Johnson CD, Kemmler G, Tomaszewski KA, Waldmann A, Young TE, Rose M (2019) General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur J Cancer 107:153–163. https://doi.org/10.1016/j.ejca.2018.11.024
Hinz A, Singer S, Brähler E (2014) European reference values for the quality of life questionnaire EORTC QLQ-C30: results of a German investigation and a summarizing analysis of six European general population normative studies. Acta Oncol 53:958–965. https://doi.org/10.3109/0284186X.2013.879998
Finck C, Barradas S, Singer S, Zenger M, Hinz A (2012) Health related quality of life in Colombia: reference values of the EORTC QLQ-C30. Eur J Cancer Care 21(6):829–836. https://doi.org/10.1111/ecc.12000
Mercieca-Bebber R, Costa DSJ, Norman R, Janda M, Smith DP, Grimison P, Gamper E, King MT (2019) The EORTC Quality of Life Questionnaire for cancer patients (QLQ-C30). Med J Aust 210(11):499–506. https://doi.org/10.5694/mja2.50207
World Population Review (2019) Human Development Index (HDI) by country 2019. http://worldpopulationreview.com/countries/hdi-by-country. Accessed 8 Nov 2019
Tadele N (2015) Evaluation of quality of life of adult cancer patients attending Tikur Anbessa specialized referral hospital, Addis Ababa Ethiopia. Ethiop J Health Sci 25(1):53–62. https://doi.org/10.4314/ejhs.v25i1.8
Ayana BA, Negash S, Yusuf L, Tigeneh W, Haile D (2016) Reliability and validity of Amharic version of EORTC QLQ-C30 questionnaire among gynecological cancer patients in Ethiopia. PLoS One 11(6):e0157359. https://doi.org/10.1371/journal.pone.0157359
Abegaz TM, Ayele AA, Gebresillassie BM (2018) Health related quality of life of cancer patients in Ethiopia. J Oncol 2018:1467595. https://doi.org/10.1155/2018/1467595
Sibhat SG, Fenta TG, Sander B, Gebretekle GB (2019) Health-related quality of life and its predictors among patients with breast cancer at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. Health Qual Life Outcomes 17(1):165. https://doi.org/10.1186/s12955-019-1239-1
Araya LT, Gebretekle GB, Gebremariam GT, Fenta TG (2019) Reliability and validity of the Amharic version of European Organization for Research and Treatment of cervical cancer module for the assessment of health related quality of life in women with cervical cancer in Addis Ababa, Ethiopia. Health Qual Life Outcomes 17(1):13. https://doi.org/10.1186/s12955-019-1089-x
Mehnert A, Koch U, Schulz H, Wegscheider K, Weis J, Faller H, Keller M, Brahler E, Harter M (2012) Prevalence of mental disorders, psychosocial distress and need for psychosocial support in cancer patients - study protocol of an epidemiological multi-center study. BMC Psychiatry 12:70. https://doi.org/10.1186/1471-244X-12-70
Hinz A, Weis J, Faller H, Brähler E, Härter M, Keller M, Schulz H, Wegscheider K, Koch U, Geue K, Götze H, Mehnert A (2018) Quality of life in cancer patients-a comparison of inpatient, outpatient, and rehabilitation settings. Support Care Cancer 26:3533–3541. https://doi.org/10.1007/s00520-018-4211-4
Scott NW, Fayers PM, Aaronson NK, Bottomley A, Graeff A de, Groenvold M, Gundy C, Koller M, Petersen MA, Sprangers, M. A. G. on behalf of the EORTC Quality of Life Group (2008) EORTC QLQ-C30 Reference Values. EORTC Quality of Life Group, available at http://groups.eortc.be/qol/sites/default/files/img/newsletter/reference_values_manual2008.pdf, Bruxelles
Quinten C, Coens C, Ghislain I, Zikos E, Sprangers MA, Ringash J, Martinelli F, Ediebah DE, Maringwa J, Reeve BB, Greimel E, King MT, Bjordal K, Flechtner HH, Schmucker-Von KJ, Taphoorn MJ, Weis J, Wildiers H, Velikova G, Bottomley A (2015) The effects of age on health-related quality of life in cancer populations: a pooled analysis of randomized controlled trials using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 involving 6024 cancer patients. Eur J Cancer 51(18):2808–2819. https://doi.org/10.1016/j.ejca.2015.08.027
Giesinger JM, Kieffer JM, Fayers PM, Groenvold M, Petersen MA, Scott NW, Sprangers MA, Velikova G, Aaronson NK (2016) Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol 69:79–88. https://doi.org/10.1016/j.jclinepi.2015.08.007
Smets EMA, Garssen B, Bonke B, Dehaes JCJM (1995) The Multidimensional Fatigue Inventory (MFI): psychometric qualities of an instrument to assess fatigue. J Psychosom Res 39(3):315–325
Zigmond AS, Snaith RP (1983) The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 67(6):361–370
Singer S, Kuhnt S, Götze H, Hauss J, Hinz A, Liebmann A, Krauß O, Lehmann A, Schwarz R (2009) Hospital Anxiety and Depression Scale cut-off scores for cancer patients in acute care. Br J Cancer 100(6):908–912. https://doi.org/10.1038/sj.bjc.6604952
Masika GM, Wettergren L, Kohi TW, Lv E (2012) Health-related quality of life and needs of care and support of adult Tanzanians with cancer. Health Qual Life Outcomes 10:133. https://doi.org/10.1186/1477-7525-10-133
Ayana BA, Negash S, Yusuf L, Tigeneh W, Haile D (2018) Health related quality of life of gynaecologic cancer patients attending at Tikur Anbesa specialized hospital (TASH), Addis Ababa, Ethiopia. BMC Womens Health 18(1):7. https://doi.org/10.1186/s12905-017-0507-7
Hinz A, Mehnert A, Dégi C, Reissmann DR, Schotte D, Schulte T (2017) The relationship between global and specific components of quality of life, assessed with the EORTC QLQ-C30 in a sample of 2019 cancer patients. Eur J Cancer Care 26(2):e12416. https://doi.org/10.1111/ecc.12416
ACTION Study Group (2017) Health-related quality of life and psychological distress among cancer survivors in Southeast Asia. BMC Med 15(1):10. https://doi.org/10.1186/s12916-016-0768-2
Subramaniam S, Kong Y-C, Chinna K, Kimman M, Ho Y-Z, Saat N, Malik RA, Taib NA, Abdullah MM, Lim GC-C, Tamin N-SI, Woo Y-L, Chang K-M, Goh P-P, Yip C-H, Bhoo-Pathy N (2018) Health-related quality of life and psychological distress among cancer survivors in a middle-income country. Psycho-Oncology 27(9):2172–2179. https://doi.org/10.1002/pon.4787
Hung H-C, Chien T-W, Tsay S-L, Hang H-M, Liang S-Y (2013) Patient and clinical variables account for changes in health- related quality of life and symptom burden as treatment outcomes in colorectal cancer. Asian Pac J Cancer Prev 14(3):1905–1909. https://doi.org/10.7314/APJCP.2013.14.3.1905
Scott NW, Fayers PM, Aaronson NK, Bottomley A, de Graeff A, Groenvold M, Koller M, Petersen MA, Sprangers MAG (2008) The relationship between overall quality of life and its subdimensions was influenced by culture: analysis of an international database. J Clin Epidemiol 61(8):788–795. https://doi.org/10.1016/j.jclinepi.2007.08.015
Bower JE (2014) Cancer-related fatigue—mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 11(10):597–609. https://doi.org/10.1038/nrclinonc.2014.127
Acknowledgments
We thank the patients, the study assistants who collected the data, and Mr. Mesfin Assefa who helped us in supervising the data collection.
Funding
Open Access funding provided by Projekt DEAL. The German study was funded by a grant from the German Cancer Aid (Grant No. 107465) within the psychosocial oncology funding priority program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the ethics committee of the University of Gondar, Ethiopia, and the ethics committees of all participating German centers.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wondie, Y., Hinz, A. Quality of life among Ethiopian cancer patients. Support Care Cancer 28, 5469–5478 (2020). https://doi.org/10.1007/s00520-020-05398-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05398-w