Abstract
Background
Invasive fungal infection (IFI) causes high morbidity and mortality during acute myeloid leukemia (AML) treatment. Interventions to prevent fungal infection, including air filtration systems and antifungal prophylaxis, may improve outcomes in this group of patients. However, they are expensive and therefore inapplicable in resource-limited countries. The benefit of antifungal therapy is also dependent on the local epidemiology. That led us to conduct the study to evaluate the characteristics and impact of IFI in AML patients without prophylaxis in our setting.
Methods
Clinical data from patients with AML who have been treated with chemotherapy without antifungal prophylaxis were retrieved during a 5-year period at Thailand’s hematology referral center. Incidence and risk factors of IFI and outcomes of patients were evaluated.
Results
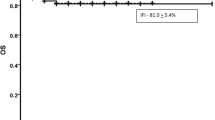
Among 292 chemotherapy courses, there were 65 (22.3%) episodes of IFI. Of those, 10 (15.4%) were proven, 19 (29.2%) were probable, and 36 (55.4%) were categorized as being possible IFI. Molds were the most commonly observed causative pathogens (93.1%). The incidence of probable/proven IFI was highest during first induction (20.5%), followed by second induction (6.1%), and consolidation (2.7%). A long duration of neutropenia, old age, and low serum albumin were the strongest predictors of IFI. Compared with patients who had no IFI, patients with probable/proven IFI had a longer length of hospital stay and higher in-hospital mortality. Patients with proven IFI had a significantly worse outcome at 1 year.
Conclusions
These results suggest the change in health policy to implement IFI preventive measures to improve outcomes of AML treatment.


Similar content being viewed by others
Change history
18 June 2019
Incorrect family name of Warissara Jutidamrongphan.
References
Pagano L, Caira M, Picardi M, Candoni A, Melillo L, Fianchi L, Offidani M, Nosari A (2007) Invasive aspergillosis in patients with acute leukemia: update on morbidity and mortality--SEIFEM-C report. Clin Infect Dis 44(11):1524–1525. https://doi.org/10.1086/517849
Even C, Bastuji-Garin S, Hicheri Y, Pautas C, Botterel F, Maury S, Cabanne L, Bretagne S, Cordonnier C (2011) Impact of invasive fungal disease on the chemotherapy schedule and event-free survival in acute leukemia patients who survived fungal disease: a case-control study. Haematologica 96(2):337–341. https://doi.org/10.3324/haematol.2010.030825
Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D (2007) Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 356(4):348–359. https://doi.org/10.1056/NEJMoa061094
Ananda-Rajah MR, Grigg A, Downey MT, Bajel A, Spelman T, Cheng A, Thursky KT, Vincent J, Slavin MA (2012) Comparative clinical effectiveness of prophylactic voriconazole/posaconazole to fluconazole/itraconazole in patients with acute myeloid leukemia/myelodysplastic syndrome undergoing cytotoxic chemotherapy over a 12-year period. Haematologica. 97(3):459–463. https://doi.org/10.3324/haematol.2011.051995
Hahn T, Cummings KM, Michalek AM, Lipman BJ, Segal BH, McCarthy PL Jr (2002) Efficacy of high-efficiency particulate air filtration in preventing aspergillosis in immunocompromised patients with hematologic malignancies. Infect Control Hosp Epidemiol 23(9):525–531. https://doi.org/10.1086/502101
Eckmanns T, Ruden H, Gastmeier P (2006) The influence of high-efficiency particulate air filtration on mortality and fungal infection among highly immunosuppressed patients: a systematic review. J Infect Dis 193(10):1408–1418. https://doi.org/10.1086/503435
Halpern AB, Lyman GH, Walsh TJ, Kontoyiannis DP, Walter RB (2015) Primary antifungal prophylaxis during curative-intent therapy for acute myeloid leukemia. Blood. 126(26):2790–2797. https://doi.org/10.1182/blood-2015-07-627323
Tang JL, Kung HC, Lei WC, Yao M, Wu UI, Hsu SC, Lin CT, Li CC, Wu SJ, Hou HA, Chou WC, Huang SY, Tsay W, Chen YC, Chen YC, Chang SC, Ko BS, Tien HF (2015) High incidences of invasive fungal infections in acute myeloid leukemia patients receiving induction chemotherapy without systemic antifungal prophylaxis: a prospective observational study in Taiwan. PLoS One 10(6):e0128410. https://doi.org/10.1371/journal.pone.0128410
Lien MY, Chou CH, Lin CC, Bai LY, Chiu CF, Yeh SP, Ho MW (2018) Epidemiology and risk factors for invasive fungal infections during induction chemotherapy for newly diagnosed acute myeloid leukemia: a retrospective cohort study. PLoS One 13(6):e0197851. https://doi.org/10.1371/journal.pone.0197851
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T et al (2008) Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46(12):1813–1821. https://doi.org/10.1086/588660
Girmenia C, Micozzi A, Piciocchi A, Gentile G, Di Caprio L, Nasso D et al (2014) Invasive fungal diseases during first induction chemotherapy affect complete remission achievement and long-term survival of patients with acute myeloid leukemia. Leuk Res 38(4):469–474. https://doi.org/10.1016/j.leukres.2014.01.007
Dragonetti G, Criscuolo M, Fianchi L, Pagano L (2017) Invasive aspergillosis in acute myeloid leukemia: are we making progress in reducing mortality? Med Mycol 55(1):82–86. https://doi.org/10.1093/mmy/myw114
Korula A, Abraham A, Abubacker FN, Viswabandya A, Lakshmi KM, Abraham OC, Rupali P, Varghese GM, Michael JS, Srivastava A, Mathews V, George B (2017) Invasive fungal infection following chemotherapy for acute myeloid leukaemia-experience from a developing country. Mycoses. 60(10):686–691. https://doi.org/10.1111/myc.12646
Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, Pastore D, Picardi M, Bonini A, Chierichini A, Fanci R, Caramatti C, Invernizzi R, Mattei D, Mitra ME, Melillo L, Aversa F, van Lint M, Falcucci P, Valentini CG, Girmenia C, Nosari A (2006) The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 91(8):1068–1075
Gheith S, Saghrouni F, Bannour W, Ben Youssef Y, Khelif A, Normand AC, Ben Said M, Piarroux R, Njah M, Ranque S (2014) Characteristics of invasive aspergillosis in neutropenic haematology patients (Sousse, Tunisia). Mycopathologia. 177(5–6):281–289. https://doi.org/10.1007/s11046-014-9742-8
Hsu LY, Lee DG, Yeh SP, Bhurani D, Khanh BQ, Low CY et al (2015) Epidemiology of invasive fungal diseases among patients with haematological disorders in the Asia-Pacific: a prospective observational study. Clin Microbiol Infect 21(6):594 e7–594 11. https://doi.org/10.1016/j.cmi.2015.02.019
Gopal S, Wood WA, Lee SJ, Shea TC, Naresh KN, Kazembe PN, Casper C, Hesseling PB, Mitsuyasu RT (2012) Meeting the challenge of hematologic malignancies in sub-Saharan Africa. Blood. 119(22):5078–5087. https://doi.org/10.1182/blood-2012-02-387092
Xu XH, Zhang L, Cao XX, Li J, Zhang W, Zhu TN, Cai HC, Chen M, Han X, Yang C, Han B, Zhang Y, Zhuang JL, Zhou DB, Duan MH (2017) Evaluation of the implementation rate of primary antifungal prophylaxis and the prognosis of invasive fungal disease in acute leukemia patients in China. J Infect Chemother 23(6):360–367. https://doi.org/10.1016/j.jiac.2017.02.011
Chen CY, Sheng WH, Tien FM, Lee PC, Huang SY, Tang JL, Tsay W, Tien HF, Hsueh PR (2018) Clinical characteristics and treatment outcomes of pulmonary invasive fungal infection among adult patients with hematological malignancy in a medical centre in Taiwan, 2008–2013. J Microbiol Immunol Infect. https://doi.org/10.1016/j.jmii.2018.01.002
Nucci M, Garnica M, Gloria AB, Lehugeur DS, Dias VC, Palma LC et al (2013) Invasive fungal diseases in haematopoietic cell transplant recipients and in patients with acute myeloid leukaemia or myelodysplasia in Brazil. Clin Microbiol Infect 19(8):745–751. https://doi.org/10.1111/1469-0691.12002
Kumar J, Singh A, Seth R, Xess I, Jana M, Kabra SK (2018) Prevalence and predictors of invasive fungal infections in children with persistent febrile neutropenia treated for acute leukemia - a prospective study. Indian J Pediatr 85(12):1090–1095. https://doi.org/10.1007/s12098-018-2722-0
Phikulsod P, Suwannawiboon B, Chayakulkeeree M (2017) Invasive fungal infection among febrile patients with chemotherapy-induced neutropenia in Thailand. Southeast Asian J Trop Med Public Health 48(1):159–169
Neofytos D, Lu K, Hatfield-Seung A, Blackford A, Marr KA, Treadway S, Ostrander D, Nussenblatt V, Karp J (2013) Epidemiology, outcomes, and risk factors of invasive fungal infections in adult patients with acute myelogenous leukemia after induction chemotherapy. Diagn Microbiol Infect Dis 75(2):144–149. https://doi.org/10.1016/j.diagmicrobio.2012.10.001
Hammond SP, Marty FM, Bryar JM, DeAngelo DJ, Baden LR (2010) Invasive fungal disease in patients treated for newly diagnosed acute leukemia. Am J Hematol 85(9):695–699. https://doi.org/10.1002/ajh.21776
Morris AL, Naeem M, Murray T, Sen J, Thomas T, Daniels E et al. (2018) Establishing an antifungal program to reduce invasive fungal infections in patients with acute myeloid leukemia receiving induction and reinduction chemotherapy. J Oncol Pract :JOP1800307. https://doi.org/10.1200/JOP.18.00307
Lewis G, Hall P, Eisa N, Deremer D, Dobbins R, El-Geneidy M et al (2010) Acute myelogenous leukemia patients are at low risk for invasive fungal infections after high-dose cytarabine consolidations and thus do not require prophylaxis. Acta Haematol 124(4):206–213. https://doi.org/10.1159/000321504
Wang L, Hu J, Sun Y, Huang H, Chen J, Li J, Ma J, Li J, Liang Y, Wang J, Li Y, Yu K, Hu J, Jin J, Wang C, Wu D, Xiao Y, Huang X Does high-dose cytarabine cause more fungal infection in patients with acute myeloid leukemia undergoing consolidation therapy: a multicenter, prospective, observational study in China. Medicine (Baltimore) 2016;95(4):e2560. doi:https://doi.org/10.1097/MD.0000000000002560
Keighley CL, Manii P, Larsen SR, van Hal S (2017) Clinical effectiveness of itraconazole as antifungal prophylaxis in AML patients undergoing intensive chemotherapy in the modern era. Eur J Clin Microbiol Infect Dis 36(2):213–217. https://doi.org/10.1007/s10096-016-2780-z
Zhang J, Liu Y, Nie X, Yu Y, Gu J, Zhao L (2018) Trough concentration of itraconazole and its relationship with efficacy and safety: a systematic review and meta-analysis. Infect Drug Resist 11:1283–1297. https://doi.org/10.2147/IDR.S170706
Glasmacher A, Prentice A, Gorschluter M, Engelhart S, Hahn C, Djulbegovic B et al (2003) Itraconazole prevents invasive fungal infections in neutropenic patients treated for hematologic malignancies: evidence from a meta-analysis of 3,597 patients. J Clin Oncol 21(24):4615–4626. https://doi.org/10.1200/JCO.2003.04.052
Rely K, Alexandre PK, Escudero GS (2011) Cost effectiveness of posaconazole versus fluconazole/itraconazole in the prophylactic treatment of invasive fungal infections in Mexico. Value Health 14(5 Suppl 1):S39–S42. https://doi.org/10.1016/j.jval.2011.05.032
Tuon FFB, Lino Florencio K, da Cunha CA, Lopes Rocha JL (2018) Cost-effectiveness of posaconazole in private and public Brazilian hospitals. Rev Iberoam Micol 35(2):63–67. https://doi.org/10.1016/j.riam.2017.09.006
Junjarunee S, Numuang K, Lerdlitruangsin S, Itzler R (2014) Cost-effectiveness of posaconazole versus fluconazole or itraconazole in the prophylaxis of invasive fungal infections among neutropenic patients in Thailand. Value Health 17(7):A806. https://doi.org/10.1016/j.jval.2014.08.522
White PL, Wingard JR, Bretagne S, Loffler J, Patterson TF, Slavin MA et al (2015) Aspergillus polymerase chain reaction: systematic review of evidence for clinical use in comparison with antigen testing. Clin Infect Dis 61(8):1293–1303. https://doi.org/10.1093/cid/civ507
Jordanides NE, Allan EK, McLintock LA, Copland M, Devaney M, Stewart K et al (2005) A prospective study of real-time panfungal PCR for the early diagnosis of invasive fungal infection in haemato-oncology patients. Bone Marrow Transplant 35(4):389–395. https://doi.org/10.1038/sj.bmt.1704768
Kourkoumpetis TK, Fuchs BB, Coleman JJ, Desalermos A, Mylonakis E (2012) Polymerase chain reaction-based assays for the diagnosis of invasive fungal infections. Clin Infect Dis 54(9):1322–1331. https://doi.org/10.1093/cid/cis132
Valero C, de la Cruz-Villar L, Zaragoza O, Buitrago MJ (2016) New panfungal real-time PCR assay for diagnosis of invasive fungal infections. J Clin Microbiol 54(12):2910–2918. https://doi.org/10.1128/JCM.01580-16
Alonso M, Escribano P, Guinea J, Recio S, Simon A, Pelaez T, Bouza E, Garcia de Viedma D (2012) Rapid detection and identification of Aspergillus from lower respiratory tract specimens by use of a combined probe-high-resolution melting analysis. J Clin Microbiol 50(10):3238–3243. https://doi.org/10.1128/JCM.00176-12
Didehdar M, Khansarinejad B, Amirrajab N, Shokohi T (2016) Development of a high-resolution melting analysis assay for rapid and high-throughput identification of clinically important dermatophyte species. Mycoses. 59(7):442–449. https://doi.org/10.1111/myc.12492
Arancia S, Sandini S, De Bernardis F, Fortini D (2011) Rapid, simple, and low-cost identification of Candida species using high-resolution melting analysis. Diagn Microbiol Infect Dis 69(3):283–285. https://doi.org/10.1016/j.diagmicrobio.2010.10.003
Blot SI, Taccone FS, Van den Abeele AM, Bulpa P, Meersseman W, Brusselaers N et al (2012) A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med 186(1):56–64. https://doi.org/10.1164/rccm.201111-1978OC
Nucci M, Anaissie E (2014) How we treat invasive fungal diseases in patients with acute leukemia: the importance of an individualized approach. Blood. 124(26):3858–3869. https://doi.org/10.1182/blood-2014-04-516211
Caira M, Candoni A, Verga L, Busca A, Delia M, Nosari A, Caramatti C, Castagnola C, Cattaneo C, Fanci R, Chierichini A, Melillo L, Mitra ME, Picardi M, Potenza L, Salutari P, Vianelli N, Facchini L, Cesarini M, de Paolis MR, di Blasi R, Farina F, Venditti A, Ferrari A, Garzia M, Gasbarrino C, Invernizzi R, Lessi F, Manna A, Martino B, Nadali G, Offidani M, Paris L, Pavone V, Rossi G, Spadea A, Specchia G, Trecarichi EM, Vacca A, Cesaro S, Perriello V, Aversa F, Tumbarello M, Pagano L, on behalf of the SEIFEM Group (Sorveglianza Epidemiologica Infezioni Fungine in Emopatie Maligne) (2015) Pre-chemotherapy risk factors for invasive fungal diseases: prospective analysis of 1,192 patients with newly diagnosed acute myeloid leukemia (SEIFEM 2010-a multicenter study). Haematologica. 100(2):284–292. https://doi.org/10.3324/haematol.2014.113399
Hoenigl M, Strenger V, Buzina W, Valentin T, Koidl C, Wolfler A, Seeber K, Valentin A, Strohmeier AT, Zollner-Schwetz I, Raggam RB, Urban C, Lass-Florl C, Linkesch W, Krause R (2012) European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) host factors and invasive fungal infections in patients with haematological malignancies. J Antimicrob Chemother 67(8):2029–2033. https://doi.org/10.1093/jac/dks155
Eriksson KM, Cederholm T, Palmblad JE (1998) Nutrition and acute leukemia in adults: relation between nutritional status and infectious complications during remission induction. Cancer. 82(6):1071–1077
Funding
This research was supported by Siriraj Cancer Foundation (Grant number 088/2558). None of the authors has financial relationship with the foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All authors declare no personal or professional conflicts of interest and no financial support from the companies that produce and/or distribute the drugs, devices, or materials described in this report. The protocol for this study was approved by the Siriraj Institutional Review Board (SIRB) of the Faculty of Medicine Siriraj Hospital, Mahidol University (COA no. 212/2016). The requirement for written informed consent was waived for this study due to its anonymous retrospective design.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nganthavee, V., Phutthasakda, W., Atipas, K. et al. High incidence of invasive fungal infection during acute myeloid leukemia treatment in a resource-limited country: clinical risk factors and treatment outcomes. Support Care Cancer 27, 3613–3622 (2019). https://doi.org/10.1007/s00520-019-04720-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04720-5