Abstract
Purpose
Breast cancer is the most common cancer of Finnish women. Peer support could be a way to help breast cancer patients to deal with the disease but studies on its effectiveness have produced conflicting results. The aim of this randomized controlled trial was to study the effectiveness of peer support on health-related quality of life (HRQoL) of breast cancer patients.
Methods
Patients with recently diagnosed breast cancer at the Helsinki University Hospital were randomly allocated to intervention (n = 130) or control (n = 130) groups. The intervention group patients received peer support via telephone one to five times according to their preference. The control group received usual care only. HRQoL was assessed with generic (15D) and disease-specific (EORTC QLQ-30 and its breast cancer specific module BR23) instruments at baseline and at 3-, 6-, and 12-month follow-up points.
Results
The mean (SD) age of the patients was 60.0 (10.5) years and their baseline mean 15D score 0.922 (0.066). At baseline, the intervention and control groups did not differ from each other. During follow-up, the 15D score deteriorated statistically significantly (p < 0.001) and clinically importantly in both groups but slightly less in the intervention group although the difference was not significant. Regarding individual 15D dimensions, the EORTC-QLQ30, or its breast-specific module, peer support did not show any consistent advantage compared to usual care.
Conclusion
Peer support had no clear effect on the HRQoL of breast cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer of Finnish women. Every year, almost 5000 women are diagnosed with breast cancer in Finland. Due to early diagnosis and effective care, about 90% of breast cancer patients are alive 5 years after diagnosis [1]. Many breast cancer patients suffer from depression and anxiety after diagnosis [2]. The efficacy of care is related to effective treatments but also to the quality of care experienced by the patient [3]. In addition to survival, quality of life is considered an important outcome of good care [4].
Quality of life is a condition that depends on changes in a person’s life such as one’s social life, health, job, and individual values. Health-related quality of life (HRQoL) includes both physical and mental perceptions of health and aspects that have an impact on them [5]. Many theories of HRQoL are based on the health definition of the World Health Organization (WHO), where health is most often seen as physical, psychological, and social wellbeing [6].
In stressful life situations, a sense of coherence can help maintain quality of life [7], but people also need social support [2]. Social support can be offered by a peer who has experienced a similar life situation [8]. Peer support consists of open discussion combined with empathy, equality, knowledge, and humor [9].
There are conflicting results concerning the effects of peer support in the earlier research. One of the reasons for the contradictory results may be the fact that peer support is a fairly wide concept, and peer support programs differ from each other. Some are led by professionals [10, 11] and some by trained volunteers [10, 12, 13]. Some of the programs are limited to peer support only [14,15,16], whereas others may have an educational component in addition to peer support [10, 12, 17], or they may be based on cultural groups [18].
According to some earlier research, face-to-face peer support groups improved wellbeing [10] and fostered personal development [19]. Also, personal peer support contact has been reported to improve wellbeing [12, 15], prepare for recovery [12], and increase self-efficacy [14, 17]. Professionally led peer support groups with health education improved physical, emotional, and social wellbeing as well [10]. Peer support has also been associated with more life purpose, less depression [10, 11, 17], fewer breast cancer concerns [17], preserved marital adjustment [15], and better future perspective [17].
However, not all studies have found positive effects of peer support on quality of life. For instance, Gotay et al. [16] found no differences in distress or depressive symptoms between peer-supported and peer-non-supported breast cancer women. Likewise, Giese-Davis et al. [15] reported that peer support did not affect trauma symptoms, self-efficacy, or depressive symptoms. In a study by Lee et al. [14], peer support had no effect on anxiety, depression, or mental adjustment and, in a study by Nápoles et al. [17], on depression or somatization.
As the results concerning the effectiveness of peer support are contradictory, it is difficult to say which method of peer support is the most effective with breast cancer. Consequently, further studies are still warranted. This study explored the effectiveness of a simple and low-cost peer support program on the HRQoL of breast cancer patients. The peer support was provided by trained volunteer breast cancer survivors via telephone and it did not include any formal rules for defining the subject and duration of discussions, or the number of phone calls between the patient and the support person. Thus, the meaning was to enable newly diagnosed breast cancer patients a simple and uncomplicated way to get in contact with survivors to be able to ask questions, and when needed, to receive emotional support.
Methods
Inclusion criteria and recruitment of participants
The participants (N = 260) were recently diagnosed primary breast cancer patients with no other serious illnesses and who were able to communicate in Finnish or Swedish (official languages in Finland), and to fill in the questionnaires. Based on the assumption that peer support would lead to a clinically important 15D score difference between the groups (0.015), we estimated that we would need 167 patients to show a significant difference with a type 1 (alpha) error rate 0.05 and power 0.80. Assuming a drop-out rate of 33%, the final sample size needed to show a significant difference was estimated to be 252.
The recruitment lasted from May 2014 to June 2015. Participants were recruited by the breast care nurses of the breast surgery unit of the Helsinki University Hospital when they came to their first appointment to the outpatient clinic. All patients meet a breast care nurse during their first outpatient clinic visit at the breast surgery unit. They also have an opportunity for further appointments or telephone calls thereafter, if required. Breast care nurses support patients and discuss the matters which are worrying patients and give them information about breast cancer and its care, social benefits, and possibilities how to get peer support. The breast care nurses told the patients about the research and gave them an information leaflet, informed consent form, and the baseline questionnaire. Women then left for home and had time to read about the research and make an informed decision whether to participate or not. If a patient wanted to participate, she filled in the informed consent form and baseline questionnaire and sent them to the hospital research secretary by mail in a prepaid envelope.
Peer support intervention
The patients were randomly allocated to an intervention (n = 130) or control (n = 130) group. In addition to usual care, the participants in the intervention group received peer support via telephone one to five times according to their preference. Peer support persons (n = 15) were breast cancer survivors trained in giving peer support. All peer support persons had breast cancer more than 2 years earlier. One third of them were still on working life, others were retired. Peer Support Training Program (contents: (1) own experience of breast cancer, (2) theory and practice of peer support, (3) confronting other patients, (4) own well-being as a peer supporter) consisted of 2 days (16 h). The control group received usual care only.
For ethical reasons, participants in the control group were not discouraged from seeking peer support by themselves if they felt a need for it. Peer support was started at the time between diagnosis and the beginning of treatments and continued according to the patients’ preferences as long as it was necessary, with approximately one call per week.
Randomization
Randomization was performed with the use of opaque sealed envelopes prepared by the lead author in groups of 20 envelopes (10 peer support and 10 control) to ascertain that the peer support persons would not be too busy in giving peer support. A hospital secretary not involved in the treatment of the patients or the assessment of treatment results performed the randomization. When she received the baseline questionnaire from a patient, she opened the numbered envelope starting from number one and ending in number 260. The researchers did not participate in the randomization.
After randomization, the names and phone numbers of patients randomized to the intervention group were forwarded via the researcher to one of the peer support persons who then contacted the patient by telephone. The number of telephone conversations between the patient and the peer support person was suggested to be between 1 and 5 according to the patient’s preference. After every call, the patient chose whether she wanted another call.
Measures
Baseline demographic variables
Demographic information concerning age, marital status, the number of children, educational level, employment status, and professional status was collected by a questionnaire devised for the study.
Primary outcome measures
HRQoL was assessed with both a generic (15D) and a disease-specific (EORTC QLQ-30 and its breast cancer specific module BR23) instrument at baseline and 3, 6, and 12 months later. In the case a patient failed to return the follow-up questionnaires, a reminder was sent.
15D
The 15D is a validated, generic, and self-administered instrument. The 15D questionnaire includes 15 dimensions: moving, seeing, hearing, breathing, sleeping, eating, speech, excretion, usual activities, mental function, discomfort and symptoms, depression, distress, vitality, and sexual activity [20]. The patient chooses a value between 1 and 5 for every dimension which describes best her current state of health (best level = 1; worst level = 5). These original values are then transformed into values from 0 to 1 for every dimension and into the total 15D score. The total 15D score represents the HRQoL of the subject with value 1 describing the best possible situation (no problems on any dimension) and 0 the worst (equivalent to being dead). The minimum clinically important difference in the 15D score, that is, the difference people can on average feel, has been estimated to be ± 0.015 [21].
EORTC QLQ-30 and its breast cancer-specific module BR23
The EORTC QLQ-C30 is a questionnaire developed by the European Organization for Research and Treatment of Cancer to assess the quality of life of cancer patients. It consists of functional (health condition, and physical, mental, psychological, and social functions) and symptomatic scales (fatigue, sickness, vomiting, pain, dyspnea, insomnia, loss of appetite, constipation, diarrhea, and economic problems). The disease-specific module for breast cancer was also used. It includes measures of mental conception of the body, sexual function, sexual function satisfaction, attitude toward the future, side effects of systemic treatments, side effects in breast and hand, and worries about hair loss [22].
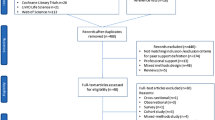
The progression of the study is shown in Fig. 1.
Ethical considerations
The research was approved by the Helsinki University Central Hospital Ethics Committee (number 178/13/03/02/2013). All patients gave written informed consent before participating in the study.
Data analysis
Statistical analysis was performed using the SPSS for Windows statistical software version 22 (SPSS, Inc., Chicago, IL, USA). The statistical significance of the change in the mean HRQoL score was tested by paired sample t test and the groups were compared using Chi-square test or the independent samples t test where appropriate. The analysis was based on the intention to treat, and two-sided p values < 0.05 were considered statistically significant.
Results
Recruitment and retention
Between May 2014 and June 2015, 850 women with breast cancer were asked to participate and given the questionnaires. Of them, 260 (30.6%) agreed by returning the questionnaires and the consent forms. Of the baseline respondents, 128 in the intervention group, and 125 in the control group, provided adequately filled 15D questionnaires that could be used for analysis. All included patients were followed up on for a year and data gathering ended in September 2016. Of the 260 enrolled patients, 91.5% also answered the 3-month, 93.1% the 6-month, and 91.2% the 12-month questionnaire, respectively. None of the participants died during the follow-up, but one patient withdrew from the study after randomization.
There were 15 trained peer support persons. Each of them contacted a variable number (mean 8.7, median 7, range 3–26) of women with breast cancer.
Baseline comparisons
The mean (SD) age of the participants was 60.0 (10.5) years. There were no significant differences between the intervention and control groups at baseline for the demographic variables recorded (Table 1).
Neither the baseline 15D score (0.922 vs. 0.921) nor any of the baseline dimension level values differed statistically significantly between the intervention and control groups (Fig. 2). The baseline EORTC-QOL and EORTC-QLQ-BR23 scores were similar in both groups, but for the EORTC-QLQ-BR23, the intervention group had slightly but statistically significantly (p < 0.05) more breast and arm symptoms (Fig. 3).
Follow-up
At the 3-month follow-up, the 15D score had deteriorated statistically significantly (p < 0.001) and clinically importantly in the whole sample from 0.922 (SD 0.066) to 0.886 (0.086). The decrease was smaller in the intervention group (0.029) than in the control group (0.044) but the difference (0.0145) did not reach statistical significance (Fig. 4). In the individual dimensions, there were no statistically significant differences between the groups although the intervention group patients fared slightly better on some dimensions (Fig. 5). During the follow-up, the small differences in some of the dimensions seen at 3 months between the groups had disappeared (data not shown).
There were no consistent meaningful differences between the groups in the EORTC-QOL30 and EORTC-QLQ-BR23 results during follow-up, although the control group was doing slightly, and statistically significantly (p < 0.05), better than the intervention group in sexual functioning at the-6-month follow-up point (data not shown).
Discussion
The aim of this study was to explore the effectiveness of a light and easily implementable peer support program on the HRQoL of breast cancer patients. The main result was that women with newly diagnosed breast cancer who were offered peer support by a breast cancer survivor had a slightly smaller deterioration in their overall HRQoL during the first 3 months of follow-up. The difference between the groups approached the minimal clinically important difference (MCID) reported for the 15D, but it did not reach statistical significance. In addition, at 3 months, the intervention group patients were slightly better off on some of the 15D dimensions (mental function, discomfort and symptoms, depression, distress), but again the differences were not statistically significant. During further follow-up, even the small differences between the groups seen during the initial months of follow-up disappeared.
The baseline mean 15D score of all the participants 0.922 (SD 0.066) was similar to that found in the general Finnish female population of approximately the same age [23]. The baseline measurement in this study was made after the women had been given a diagnosis of breast cancer. After 3 months, at the time of the first follow-up, patients had already undergone a breast cancer operation and possible medical treatment had started. At that time, HRQoL had deteriorated in both groups but slightly less in those who had received peer support.
The intervention in this study was provided soon after the women received the diagnosis of breast cancer, and it was intended to consist of one to five phone calls during the first weeks after diagnosis. Some of the women in the intervention group did not want to have a long discussion with the peer support person, even though they were in contact by telephone. One woman in the intervention group was not contacted because she did not reveal her telephone number. Despite this, the analyses were performed on an intention-to-treat basis. If the intervention had lasted longer, the effect might have been clearer, as reported by Giese-Davis et al. [16], where the intervention lasted for 6 months and the effects on quality of life were seen over 12 months.
Comparison of peer support studies is difficult because there are many variables that can influence the effectiveness of the support. These include the form and duration of peer support as well as the instruments used to assess its effectiveness. We chose to measure HRQoL with the 15D and EORTC measures because they have been used in many other studies at the Helsinki University Hospital. The EORTC was also used in a study by Sharif et al. [17] which, in contrast to ours, reported a much more favorable effect for a month-long peer support intervention on all performance aspects of quality of life. However, their peer support intervention was also more intense than ours.
The strengths of our study include the success of the randomization process, where the intervention and control groups did not differ from each other in the demographic variables recorded. Furthermore, with 260 breast cancer patients, the size of the study group was relatively large. The study was performed in an internationally recognized hospital unit specialized in the treatment of breast cancer care and the follow-up response rate was good: of the 260 women who participated in the study, up to 92% also answered the 3-month follow-up measures, 93% the 6-month follow-up, and 91% the 12-month follow-up.
A limitation of the study is the fact that we did not collect information concerning the stage of the cancer or of the treatments the participants received. Consequently, we cannot rule out their role behind the difference in deterioration of HRQoL seen at 3 months. However, in the clinical trial by Mens et al. [11] the severity of the cancer had no influence on the effects of a peer support intervention. In our study, the number of peer support contacts was mainly determined by the patients themselves according to their preference. Peer support persons reported that some of the patients wanted only one call whereas others needed more. Because we did not, unfortunately, record the number of calls each patient received, we were unable to study whether the number of calls was associated with the outcomes.
There was a high non-participation rate in this study. Breast care nurse, who recruited the participants did try to convince all the patients about the importance of peer support and the study and motivate them to participate. The non-volunteering patients were not sent any reminders. Furthermore, as those who did not participate did not provide an informed consent, we were unfortunately unable to follow them up in any way. This limits the generalizability of our findings as we cannot with certainty rule out that those interested in receiving peer support would not differ from the average breast cancer patients.
This study was conducted in a large university hospital unit specialized in breast cancer care which sees more than 1000 new breast cancer patients every year. Every patient meets, at least once prior to surgery, a breast care nurse experienced in answering any possible questions the patients might have. If the study had been conducted at another clinic, where the nurses were not as well trained to provide social support, the influence of peer support might have been greater.
Due to ethical reasons, we encouraged all patients to get into contact with patient organizations to get peer support if they felt a need for it. As breast cancer is a common disease, many women also have a chance to discuss their situation with a breast cancer survivor whom they already know. This might have had an effect on the results of this study although we do not know how many women in the control group actually received some kind of peer support.
Some important questions, however, remain. These include how the participants experienced the peer support and what their views are on developing peer support. We are currently examining these questions based on interviews with the participants who were offered support and we will report the results in a subsequent article.
Conclusion
In conclusion, in a university hospital setting specialized in breast cancer treatment, peer support had only a marginal and transient effect on breast cancer patients’ health-related quality of life.
References
Cancer Society of Finland. Cancers in 2030. https://www.cancersociety.fi/publications/reports/cancer-in-finland-2016/cancers-in-2030/. Accessed 5 Dec 2017
Reich M, Lesur A, Percrizet-Chevallier C (2008) Depression, quality of life and breast cancer: a review of the literature. Breast Cancer Res Treat 110:9–17
Sintonen H (2001) The 15D instrument of health-related quality of life: properties and applications. Ann Med 33:328–336
EORTC Quality of life. Quality of life http://groups.eortc.be/qol/quality-life. Accessed 3 Jan 2018
Centers for Disease Control and Prevention. Health-Related Quality of Life (HRQOL) (2016) https://www.cdc.gov/hrqol/concept.htm. Accessed 8 Jan 2017
Official records of the World Health Organization No 2 (1948) http://apps.who.int/iris/bitstream/10665/85573/1/Official_record2_eng.pdf. Accessed 8 Dec 2017
Lindström B, Eriksson M (2010) The hitchhiker’s guide to salutogenesis. Salutogenic pathways to health promotion. Folkhälsan Research Center. Health Promotion Research. Research report 2010:2. Folkhälsan. IUHPE
Campbell HC, Phaneuf MR, Deane K (2004) Cancer peer support programs –do they work? Patient Educ Couns 55:3–15. https://doi.org/10.1016/j.pec.2003.10.001
Pistrang N, Jay Z, Gessler S, Barker C (2012) Telephone peer support for women with gynaecological cancer: recipients’ perspectives. Pshchooncology 21(10):1082–1090
Tehrani AM, Farajzadegan Z, Rajabi FM, Zamani AR (2011) Belonging to a peer support group enhance the quality of life and adherence rate in patients affected by breast cancer: a non-randomized controlled clinical trial. J Res Med Sci 16:658–665 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3214378/. Accessed 7 Jun 2017
Mens GM, Helgeson VS, Lembersky BC, Baum A, Scheier MF (2016) Randomized psychosocial interventions for breast cancer: impact on life purpose. Psychooncology 25:618–625 www.ncbi.nlm.nih.gov/pmc/articles/PMC4945105/. Accessed 7 June, 2017
Mollica MA, Nemeth LS, Newman SD, Mueller M, Sterba K (2014) Peer navigation in African American breast cancer survivors. Dovepress 5:131–144. https://www.dovepress.com/peer-navigation-in-african-american-breast-cancer-survivors-peer-reviewed-fulltext-article-PROM. Accessed 16 Feb 2018
Power S, Hegarty J (2010) Facilitated peer support in breast cancer. A pre- and post-program evaluation of women’s expectations and experiences of women’s expectations and experienced of a facilitated peer support program. Cancer Nurs 33:E9–E16
Lee R, Lee KS, Oh EG, Kim SH (2013) A randomized trial of dyadic peer support intervention for newly diagnosed breast cancer patients in Korea. Cancer Nurs 36:E15–E22 https://journals.lww.com/cancernursingonline/Abstract/2013/05000/A_Randomized_Trial_of_Dyadic_Peer_Support.13.aspx. Accessed 1 Feb 2017
Giese-Davis J, Bliss-Isberg C, Wittenberg L, White J, Star P, Zhong L, Cordova MJ, Houston D, Spiegel D (2016) Peer-counseling for women newly diagnosed with breast cancer: a randomized community/research collaboration trial. Cancer 122:2408–2417 onlinelibrary.wiley.com/doi/10.1002/cncr.30036/full
Gotay CC, Monipour CM, Unger JM et al (2007) Impact of a peer-delivered telephone intervention for women experiencing a breast cancer recurrence. J Clin Oncol 25(16):2093–2098 ascopubs.org/doi/full/10.1200/jco.2006.07.4674. Accessed 7 Aug 2017
Sharif F, Abshorshori N, Tahmasebi S, Hazrati M, Zare N, Masoumi S (2010) The effect of peer-led education on the life-quality of mastectomy patients referred to breast cancer-clinics in Shiraz, Iran 2009. Health Qual Life Outcomes 8:74 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2919455/. Accessed 7 Aug 2017
Nápoles AM, Ortiz C, Santoyo-Olsson J et al (2015) Nuevo Amanecer: results of a randomized controlled trial of a community-based, peer-delivered stress management intervention to improve quality of life in Latinas with breast cancer. Am J Public Health 105:e55–e63 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4455521/. Accessed 7 Aug 2017
Ashing-Giwa K, Tapp C, Rosales M, McDowell K, Martin V, Santifer RH, Clark P, Steward J, Lewis L, Mitchell E (2012) Peer-based models of supportive care: the impact of peer support groups in African American breast cancer survivors. Oncol Nurs Forum 39:585–591 https://onf.ons.org/onf/39/6/peer-based-models-supportive-care-impact-peer-support-groups-african-american-breast-cancer. Accessed 7 Oct 2017
15D instrument. www.15d-instrument.net. Accessed 5 Jan 2018
Alanne S, Roine RP, Räsanen P, Vainiola T, Sintonen H (2015) Estimating the minimum important change in the 15D scores. Qual Life Res 24:599–606
EORTC Quality of life. EORTC QLQ-C30. http://groups.eortc.be/qol/eortc-qlq-c30. Accessed 3 Jan 2018
Roine E, Blomqvist C, Kellokumpu-Lehtinen PL, Sintonen H, Saarto T (2016) Health-related quality of life in breast cancer patients after adjuvant treatments. Breast J 22:473–475
Acknowledgements
We would like to warmly thank all the women who participated in the study as well as all the warm-hearted peer support persons. We would also like to thank Mr. Jari Villberg for making the power and sample size calculations and Ms. Heli Sarpila for randomizing the patients and saving the data.
Funding
Open access funding provided by University of Jyväskylä (JYU). This research was funded by the Finnish Cultural Foundation and HUS—the Hospital District of Helsinki and Uusimaa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
This article does not contain any studies with animal performed by any of the authors.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Toija, A.S., Kettunen, T.H., Leidenius, M.H.K. et al. Effectiveness of peer support on health-related quality of life in recently diagnosed breast cancer patients: a randomized controlled trial. Support Care Cancer 27, 123–130 (2019). https://doi.org/10.1007/s00520-018-4499-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4499-0