Abstract
Background
Bone metastasis is reported to be associated with poor quality of life, and increased risk of hospitalization. We aim to synthesize evidence from published randomized controlled trials (RCTs) which compared the efficacy of denosumab versus bisphosphonates in patients with advanced cancers.
Methods
We searched for all published RCTs in the following electronic databases: PubMed, Scopus, Web of Science, and Cochrane Central. Retrieved records were screened for eligibility. Time-to-event data were pooled as hazard ratio (HR) using the generic inverse-variance method and dichotomous data were pooled as relative risk (RR) in a random-effect model. We used Review Manager 5.3 for windows.
Results
Six unique RCTs with a total of 7722 patients were included. Overall effect estimates favored denosumab group in comparison to intravenous (IV) bisphosphonates in the following terms: time to first skeletal-related events (HR 0.92, 95% CI [0.86, 0.98], p = 0.01), time to subsequent skeletal-related event (RR 0.92, 95% CI [0.86, 0.99], p = 0.03), and radiation to bone (RR 0.81, 95% CI [0.71, 0.92], p = 0.02). Denosumab group was associated with increased risk of grade 3 or 4 hypocalcaemia (RR 1.99, 95% CI [1.11, 3.54], p = 0.02) and reduced risk of renal impairment or toxicity (RR 0.75, 95% CI [0.61, 0.91], p = 0.003) in comparison to IV bisphosphonates group. Pooled studies were homogenous.
Conclusion
Denosumab showed a favorable significant impact on delaying the time to first skeletal-related event and reducing the incidence of radiation to the bone event in comparison to bisphosphonates, with similar efficacy regarding overall survival and time to disease progression. Further large-scale and long-term studies are needed to clarify the long-term efficacy and safety of both regimens.
Similar content being viewed by others
Introduction
Bone metastasis commonly accompanies with malignant tumors of the breast (73%), prostate (68%), or lung (36%) [1]. It is associated with local irreversible skeletal-related events (SREs) of spinal cord compression, pathologic fracture, and radiation to bone or surgery to bone [1]. SREs are accompanied with inferior functional, physical and emotional status, humbler overall quality of life, and increased risk of hospitalization and hospital stay [2, 3]. Bone metastatic tumor cells release growth factors and cytokines that stimulate increased expression of RANK ligand that in turn promotes osteoclastic activity, causing substantial bone destruction [4]. Intravenous (IV) bisphosphonates have been the mainstay of the prevention of SREs in patients with metastatic solid tumors. They are pyrophosphate analogs; bind hydroxyapatite in bone thus inhibits osteoclast activity. However, IV bisphosphonates are contraindicated in renal impairments as they may cause renal toxicity and acute-phase reactions [5, 6].
Denosumab is a human monoclonal antibody that binds to human RANKL, inhibiting osteoclast-mediated bone destruction. A recent cost-effectiveness analysis of denosumab versus zoledronic acid (ZA) showed that denosumab was associated with a lower number of SREs, increased quality-adjusted life years (QALY), and increased lifetime total costs compared to ZA. The charges per QALY gained for denosumab versus bisphosphonates in castration-resistant prostate cancer, breast cancer, and non-small cell lung cancer were $49,405, $78,915, and $67,931, respectively, frequently considered decent value in the USA. Costs per SREs avoided were $8567, $13,557, and $10,513, respectively [7]. Moreover, recent clinical trials either demonstrate a trending superiority or non-inferiority of denosumab compared to bisphosphonates regarding SREs in patients with solid tumors [8, 9].
The present meta-analysis provides class one of evidence by pooling randomized controlled trials (RCTs) which compared the efficacy of denosumab versus bisphosphonates in preventing SREs in patients with advanced cancers.
Methods
We performed this review according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [10].
Inclusion and exclusion criteria
We included RCTs with the following criteria: (1) studies that compare the efficacy and safety of denosumab and zoledronic acid in delaying skeletal-related events, (2) studies that include patients with advanced breast cancer and other tumors, (3) reporting data on humans only, and (4) no restriction on race, place, sex, age, ethnicity, or language. In the case of multiple reports for the same study population, we analyzed data of the most complete dataset. Studies were excluded for the following reasons: (1) studies in which patients were not randomized and (2) thesis and conference papers.
Literature search strategy
We performed a comprehensive search of four electronic databases: Pubmed, Scopus, ISI Web of Science, and Cochrane Library, for relevant studies published in the literature till January 2017. The search term planned for Pubmed was the following: “denosumab AND zoledronic acid AND bone metastases”. This term was made suitable for different databases. We conducted an additional manual search for relevant studies through searching for RCTs in the references of included studies. We retrieved the results of searching the databases and removed duplicated studies by EndNote X7.4 software. The titles and abstracts of retrieved records were screened by three independent reviewers to exclude irrelevant articles and consider potentially included articles. Any disagreements were resolved by discussion and consensus was reached. We screened the full texts of potentially included studies. Three reviewers screened the full texts independently and included only the studies meeting our criteria.
Data extraction
All authors contributed to the development of an extraction form in an excel sheet. Efficacy outcomes were the following: time to first on-study skeletal-related event, time to first and subsequent on-study skeletal-related events, time to disease progression, and overall survival. Safety outcomes were as the following: any adverse event, anemia, dyspnea, anorexia, fatigue, bone pain, asthenia, arthralgia, peripheral edema, hypocalcemia, fatal adverse events, infectious adverse events, cumulative osteonecrosis of the jaw, and new primary malignant. Three reviewers independently extracted data from the included articles; any discrepancies were solved by discussion. We extracted data from graphs using Plot Digitizer software (http://plotdigitizer.sourceforge.net/).
Quality assessment
The quality of the retrieved RCTs was assessed according to Cochrane Handbook of Systematic Reviews of Interventions 5.1.0 (updated March 2011). Risk of bias assessment included the following domains: sequence generation (selection bias), allocation sequence concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other potential sources of bias. The authors’ judgments are categorized as “Low risk,” “High risk,” or “Unclear risk” of bias. We used the quality assessment table provided in (part 2, Chapter 8.5) the same book [11].
Measures of treatment effect
The primary outcome measurements, in studies comparing the efficacy and safety of denosumab and zoledronic acid, were the following: time to first on-study skeletal-related event, time to first and subsequent on-study skeletal-related events, time to disease progression, overall survival, and safety outcomes.
Dealing with missing data
In the case of missing standard deviation (SD) of mean change from baseline, it was calculated from standard error or 95% confidence interval (CI) according to Altman [12].
Data synthesis
Dichotomous data were pooled as relative risk (RR) in a random-effect model. Time-to-event data were pooled as hazard ratio (HR) using the generic inverse-variance method. We used Review Manager 5.3 for windows.
Assessment of heterogeneity
Heterogeneity was assessed by visual inspection of the forest plots and measured by I-square and chi-square tests. Chi-square test was used to test the existence of significant heterogeneity while I-square quantifies the variability in effect estimates that is due to heterogeneity, if present. I-square test was interpreted according to recommendations of Cochrane Handbook of Systematic Reviews and Meta-analysis (0 to 40%, might not be important; 30% to 60%, may represent moderate heterogeneity; 50% to 90%, may represent substantial heterogeneity; and 75% to 100%, considerable heterogeneity). Significant heterogeneity was considered at chi-square (p < 0.1).
Publication bias
According to Egger and colleagues [13, 14], publication bias assessment is not reliable for less than ten pooled studies. Therefore, in the present study, we could not assess the existence of publication bias by Egger’s test for funnel plot asymmetry.
Results
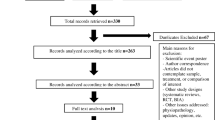
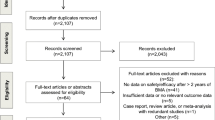
We retrieved 548 unique citations. A total of 27 publications were identified after title and abstract screening. From which, six unique RCTs with a total of 7722 patients (denosumab group n = 3984 and IV Bisphosphonates group n = 3738) were included in the present systematic review and meta-analysis (see PRISMA flow diagram; Fig. 1).
The sample size of the included trials ranged from 110 to 1900 patients. Two trials included patients with advanced breast cancer [15, 16]. One trial included patients with castration-resistant prostate cancer [9]. Two trials included patients with solid tumors (excluding breast and prostate cancers) [8, 17] and one trial included patients with carcinomas (except lung) or multiple myeloma [18]. Denosumab was administrated subcutaneously at 120 mg every 4 weeks in five included trials [8, 15, 17, 18]. Fizazi et al. administrated denosumab subcutaneously at 180 mg every 4 weeks or at 180 mg every 12 weeks for 25 weeks [18]. There was no statistically significant difference between denosumab and IV bisphosphonates regarding ECOG performance status, pain scores, or history of previous skeletal-related events. Summary of included studies and baseline characteristics is shown in Table 1.
Five included trials reported a statistically significant superiority of denosumab over IV bisphosphonates in delaying or preventing skeletal-related events in patients with bone metastases [8, 9, 15, 17, 18]. Only, Lipton et al. reported no differences between denosumab and IV bisphosphonate groups in suppressing bone turnover and skeletal-related events reducing risk [16].
The quality of the included RCTs was from moderate to high quality according to the Cochrane risk of bias assessment tool. Summary of quality assessment domains of included studies is shown in Fig. 2. Authors’ judgments with justifications are shown in supplementary file no. 1.
Effect of denosumab in comparison to IV bisphosphonates
Overall effect estimates favored denosumab group in comparison to IV bisphosphonates in the following terms: time to first skeletal-related events (HR 0.92, 95% CI [0.86, 0.98], p = 0.01; Fig. 3a), time to subsequent skeletal-related event (RR 0.92, 95% CI [0.86, 0.99], p = 0.03; Fig. 3b), and radiation to bone (RR 0.81, 95% CI [0.71, 0.92], p = 0.001; Fig. 3c). However, overall effect estimates did not favor denosumab group in comparison to IV bisphosphonates in the following terms: overall survival (HR 0.98, 95% CI [0.92, 1.06], p = 0.65; Fig. 4a), time to disease progression (HR 1, 95% CI [0.94, 1.07], p = 0.94; Fig. 4b), spinal cord compression (RR 0.84, 95% CI [0.57, 1.23], p = 0.0), surgery to bone (RR 0.63, 95% CI [0.32, 1.27], p = 0.0), and pathological fractures (RR 0.93, 95% CI [0.79, 1.10], p = 0.0). For all efficacy outcomes, the pooled effects were not heterogeneous (chi-square, p > 0.1).
Forest plots of efficacy end points for denosumab group versus bisphosphonate group. a Time to first skeletal-related events presented as hazard ratio between the two groups with 95% confidence interval. b Time to subsequent skeletal-related event presented as hazard ratio between the two groups with 95% confidence interval. c Radiation to bone presented as risk ratio between the two groups with 95% confidence interval. RR, Risk Ratio; IV, inverse variance; M-H, Mantel–Haenszel; CI, confidence interval
Forest plots of efficacy end points for denosumab group versus bisphosphonate group. a Overall survival (OS) presented as hazard ratio between the two groups with 95% confidence interval. b Progression-free survival (PFS) presented as hazard ratio between the two groups with 95% confidence interval. IV, inverse variance; M-H, Mantel–Haenszel; CI, confidence Interval
Safety outcomes
The total number and frequency of the reported adverse events did not differ significantly between the denosumab and IV bisphosphonate groups (RR 1.00, 95% CI [0.99, 1.01], p = 0.93).
The pooled RR of adverse events was as follows: fatal adverse events (RR 1.02, 95% CI [0.84, 1.23], p = 0.87), treatment discontinuation due to adverse events (RR 0.93, 95% CI [0.66, 1.31], p = 0.68), renal impairment or toxicity (RR 0.75, 95% CI [0.61, 0.91], p = 0.003), grade 3 or 4 hypocalcaemia (RR 1.99, 95% CI [1.11, 3.54], p = 0.02), anemia (RR 0.9, 95% CI [0.82, 1.00], p = 0.06), fatigue (RR 1.03, 95% CI [0.92, 1.14], p = 0.61), and asthenia (RR 0.96, 95% CI [0.87, 1.07], p = 0.47).
Discussion
Efficacy of denosumab versus bisphosphonates
The present meta-analysis demonstrated that, in the terms of the time to first skeletal-related event and time to subsequent skeletal-related event, denosumab was superior over bisphosphonates. There was no significant difference in terms of overall survival and time to disease progression between the two groups of the included studies and the present meta-analysis [8, 19, 20]. Nonetheless, these results were in concordance with the Zheng et al. [21] meta-analysis study. The overall effect estimate did not favor either of the two groups in terms of spinal cord compression, surgery to bone, and pathological fractures among the included studies, which was in concordance with the results of the present meta-analysis [8, 15, 19].
The overall effect of radiation to bone favored denosumab over bisphosphonates in the meta-analysis and Martin et al. [15] clinical trial. Conversely, it did not favor any of the two groups in both clinical trials of Fizazi et al. [19] and Henry et al. [8].
Based on these findings and previous reports [21], denosumab should be arguably considered as a first-line treatment to prevent skeletal-related events in cancer patients. In addition to its efficacy, denosumab is characterized by a more rapid onset and longer duration of action than bisphosphonates [22]. Denosumab was linked to lower risk of tachyphylaxis and osteonecrosis of the jaw as well [23, 24]. However, these mentioned advantages may be outweighed by a number of limitations which may potentially explain the limited use denosumab as the first-line treatment option. It was reported that denosumab discontinuation is followed by a marked rise of bone markers to the pretreatment level, which may limit its effectiveness [25]. Denosumab is associated with increased risk of hypocalcemia due to its powerful antiresorptive effect [8, 16, 19, 20]. Therefore, large-scale real-life studies are needed to address the potential role of denosumab as a first-line treatment option.
Another concern is the superiority of denosumab over bisphosphonates in case of hematological malignancy, especially in multiple myeloma. According to the post hoc analysis of Henry and colleagues [17], the overall survival favored ZA over denosumab in patients with multiple myeloma (HR 2.26; p = 0.014), although the overall survival did not favor either treatment in the full data set. Such findings may be explained by the ability of myeloma to inhibit osteoblasts through the secretion of DKK1 and other factors [26], while denosumab acts only against osteoclast. However, Raje et al. [27] performed a subset analysis on the multiple myeloma patients included in Henry and colleagues. They found that denosumab group had poor prognostic factors and received less effective treatment than the ZA group. Moreover, a number of high-risk patients in ZA group withdrew from the study, which may affect the reliability of the detected difference [27]. In the present meta-analysis, we could not perform a subgroup analysis according to tumor types due to lack of reported data; thus, we cannot draw a conclusion about the difference between denosumab and bisphosphonates in myeloma patients. We recommend the conduction of well-designed clinical trials to evaluate the efficacy of denosumab versus bisphosphonates in myeloma patients.
Safety of denosumab versus bisphosphonates
The main difference between denosumab and bisphosphonate is the variation in pharmacokinetic and pharmacodynamic profiles. Bisphosphonate is excreted intact primarily through the kidneys and has been associated with clinically significant nephrotoxicity, especially in ZA and to a lesser extent pamidronate (possibly including collapsing focal segmental glomerulosclerosis and acute tubular necrosis) and renal failure occasionally [28, 29]. Denosumab, fully human monoclonal antibody, excretion does not rely on renal function, as the antibody is metabolized through nonspecific catabolism in the reticuloendothelial system [30]. The international expert panel endorsed that an intravenous bisphosphonate should not be administered in combination with nephrotoxic chemotherapy [31]. In the present meta-analysis and the included clinical trials [8, 15, 20], the incidence of nephrotoxicity was higher in bisphosphonates group over denosumab group. However, it did not favor any of the two groups according to Fizazi et al. [19, 32]. Owing to the nephrotoxic profile of bisphosphonate, dosing of bisphosphonate requires renal function monitoring which is not required for denosumab. In turn, this may enforce an additional inappropriateness on numerous patients, mainly those receiving other nephrotoxic therapies. Acute-phase reactions, defined as brief immune-driven responses, usually following the first or second dose of intravenous bisphosphonates, are a recognized adverse effect of zoledronic acid, however, have not been documented to denosumab [33, 34]. Of note, hypocalcemia was more commonly encountered with denosumab over bisphosphonate according to the results of the present meta-analysis and the included studies [8, 16, 19, 20] except for the clinical trial which performed in 2009 by Fizazi et al. [32]. The predominance of hypocalcemia among the denosumab-treated group could be explained by the higher potency of denosumab over bisphosphonate as an antiresorptive agent. Therefore, it is recommended for patients on bone-targeted therapy, whether with bisphosphonates or denosumab, receive supplemental calcium and vitamin D, with the omission of those with clinical hypocalcemia [8].
The strength of the study versus the limitation of the study
The strengths of the current meta-analysis comprise a comprehensive search of published and unpublished clinical trials studies from multiple electronic databases. However, we could not include any unpublished study. Funnel plots showed asymmetrical distribution of the effect size; this could not be confirmed statistically by Egger’s test, as the number of eligible studies is < 10 studies as stated by Egger et al. [13]. Furthermore, there was a transparent assessment of the quality of evidence.
The main limitation of this meta-analysis is the small number of included studies. Consequently, we cannot judge the overall survival improvement by subgroup analysis between different advanced solid tumors with a lot of data in details. There were two included open-labeled clinical trials, which increase the risk of performance bias [16, 32]. Furthermore, the discrepancy of subgroup, i.e., the variable pathophysiology and course of each tumor, and the lack of stratification by subgroups at randomization among the included studies hamper the accuracy of results of efficacy and safety profile of both regimens. There was a high risk of bias at the funding of the trails, as all the included clinical trials in the meta-analysis were funded by drug companies.
The implication for future research
Due to the comparatively small sample size, the conclusions require further confirmation and validation. However, the high quality of the included studies among different populations such as European, Asia, Australia, North America, and South America populations improves the reliability of results. Of note, the populations from Africa were not included, and thus, we recommend perform such trail there. Therefore, we recommend the application of denosumab versus bisphosphonates regimen on long-scale clinical trials to identify the long-term efficacy and safety. Further clinical trials studies are required to study convenience of denosumab in comparison with other agents especially the safe renal profile bisphosphonate, ibandronate, used for the treatment of metastatic bone disease patients among different levels of severity.
Conclusion
Denosumab showed a favorable significant impact on delaying the time to first skeletal-related event and reducing the incidence of radiation to the bone event in comparison to bisphosphonates, with similar efficacy regarding overall survival and time to disease progression. Though nephrotoxicity was more encountered among bisphosphonate-treated patients; hypocalcemia was significantly more common among denosumab-treated patients. Further large-scale and long-term studies are needed to clarify the long-term efficacy and safety of both regimens.
References
Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res An Off J Am Assoc Cancer Res 12(20):6243s–6249s. https://doi.org/10.1158/1078-0432.CCR-06-0931
Costa L, Badia X, Chow E et al (2008) Impact of skeletal complications on patients’ quality of life, mobility, and functional independence. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 16(8):879–889. https://doi.org/10.1007/s00520-008-0418-0
Pockett RD, Castellano D, McEwan P et al (2010) The hospital burden of disease associated with bone metastases and skeletal-related events in patients with breast cancer, lung cancer, or prostate cancer in Spain. Eur J Cancer Care (Engl) 19(6):755–760. https://doi.org/10.1111/j.1365-2354.2009.01135.x
Hofbauer LC, Neubauer A, Heufelder AE (2001) Receptor activator of nuclear factor-kappaB ligand and osteoprotegerin: potential implications for the pathogenesis and treatment of malignant bone diseases. Cancer 92:460–470
Ruza I, Mirfakhraee S, Orwoll E, Gruntmanis U (2013) Clinical experience with intravenous zoledronic acid in the treatment of male osteoporosis: evidence and opinions. Ther Adv Musculoskelet Dis 5(4):182–198. https://doi.org/10.1177/1759720X13485829
Smith MR (2005) Zoledronic acid to prevent skeletal complications in cancer: corroborating the evidence. Cancer Treat Rev 31:19–25. https://doi.org/10.1016/j.ctrv.2005.09.004
Stopeck A, Rader M, Henry D et al (2012) Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J Med Econ 15(4):712–723. https://doi.org/10.3111/13696998.2012.675380
Henry D, Vadhan-Raj S, Hirsh V et al (2014) Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer 22(3):679–687. https://doi.org/10.1007/s00520-013-2022-1
Fizazi K, Carducci M, Smith M, et al (2011) Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377:813–822. doi: https://doi.org/10.1016/S0140-6736(10)62344-6, 9768
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Green S, Higgins P, T. J, Alderson P et al (2011) Cochrane Handbook: Cochrane Reviews: Ch 8: Assessing risk of bias in included studies. In: Cochrane Handb. Syst. Rev. Interv, pp 3–10
Altman DGG, Bland JMM (2005) Standard deviations and standard errors. BMJ 331(7521):903. https://doi.org/10.1136/bmj.331.7521.903
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7129):629–634. https://doi.org/10.1136/bmj.316.7129.469
Terrin N, Schmid CH, Lau J, Olkin I (2003) Adjusting for publication bias in the presence of heterogeneity. Stat Med 22(13):2113–2126. https://doi.org/10.1002/sim.1461
Martin M, Bell R, Bourgeois H et al (2012) Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res 18(17):4841–4849. https://doi.org/10.1158/1078-0432.CCR-11-3310
Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, de Boer R, Berardi R, Gascon P, Tonkin KS, Coleman R, Paterson AHG, Peterson MC, Fan M, Kinsey A, Jun S (2007) Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol 25(28):4431–4437. https://doi.org/10.1200/JCO.2007.11.8604
Henry DH, Costa L, Goldwasser F et al (2011) Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 29(9):1125–1132. https://doi.org/10.1200/JCO.2010.31.3304
Fizazi K, Lipton A, Mariette X et al (2009) Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol 27:1564–1571. https://doi.org/10.1200/JCO.2008.19.2146
Fizazi K, Carducci M, Smith M et al (2011) Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet (London, England) 377(9768):813–822. https://doi.org/10.1016/S0140-6736(10)62344-6
Henry DH, Costa L, Goldwasser F et al (2011) Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol Off J Am Soc Clin Oncol 29(9):1125–1132. https://doi.org/10.1200/JCO.2010.31.3304
Zheng GZ, Chang B, Lin FX, Xie D, Hu QX, Yu GY, du SX, Li XD (2016) Meta-analysis comparing denosumab and zoledronic acid for treatment of bone metastases in patients with advanced solid tumours. Eur J Cancer Care (Engl) doi: https://doi.org/10.1111/ecc.12541, 26, 6
Nanes MS (2010) Preventing metastases to bone: denosumab or bisphosphonates? J Bone Miner Res 25(3):437–439. https://doi.org/10.1002/jbmr.71
Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, de Boer R, Berardi R, Gascon P, Tonkin KS, Coleman RE, Paterson AHG, Gao GM, Kinsey AC, Peterson MC, Jun S (2008) Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin Cancer Res 14(20):6690–6696. https://doi.org/10.1158/1078-0432.CCR-07-5234
Coleman R, Brown J, Terpos E et al (2008) Bone markers and their prognostic value in metastatic bone disease: clinical evidence and future directions. Cancer Treat Rev 34(7):629–639. https://doi.org/10.1016/j.ctrv.2008.05.001
Miller PD, Wagman RB, Peacock M, Lewiecki EM, Bolognese MA, Weinstein RL, Ding B, Martin JS, McClung MR (2011) Effect of denosumab on bone mineral density and biochemical markers of bone turnover: six-year results of a phase 2 clinical trial. J Clin Endocrinol Metab 96(2):394–402. https://doi.org/10.1210/jc.2010-1805
Fowler JA, Mundy GR, Lwin ST, Edwards CM (2012) Bone marrow stromal cells create a permissive microenvironment for myeloma development: a new stromal role for Wnt inhibitor Dkk1. Cancer Res 72(9):2183–2189. https://doi.org/10.1158/0008-5472.CAN-11-2067
Raje N, Vadhan-Raj S, Willenbacher W, Terpos E, Hungria V, Spencer A, Alexeeva Y, Facon T, Stewart AK, Feng A, Braun A, Balakumaran A, Roodman GD (2016) Evaluating results from the multiple myeloma patient subset treated with denosumab or zoledronic acid in a randomized phase 3 trial. Blood Cancer J 6(1):e378. https://doi.org/10.1038/bcj.2015.96
Diel IJ, Bergner R, Grötz KA (2007) Adverse effects of bisphosphonates: current issues. J Support Oncol 5:475–482
Perazella MA, Markowitz GS (2008) Bisphosphonate nephrotoxicity. Kidney Int 74(11):1385–1393. https://doi.org/10.1038/ki.2008.356
Tabrizi MA, Tseng C-ML, Roskos LK (2006) Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today 11(1-2):81–88. https://doi.org/10.1016/S1359-6446(05)03638-X
Aapro M, Abrahamsson PA, Body JJ, Coleman RE, Colomer R, Costa L, Crino L, Dirix L, Gnant M, Gralow J, Hadji P, Hortobagyi GN, Jonat W, Lipton A, Monnier A, Paterson AHG, Rizzoli R, Saad F, Thurlimann B (2008) Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol Off J Eur Soc Med Oncol 19(3):420–432. https://doi.org/10.1093/annonc/mdm442
Fizazi K, Lipton A, Mariette X et al (2009) Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol Off J Am Soc Clin Oncol 27(10):1564–1571. https://doi.org/10.1200/JCO.2008.19.2146
Adami S, Bhalla AK, Dorizzi R, Montesanti F, Rosini S, Salvagno G, Lo Cascio V (1987) The acute-phase response after bisphosphonate administration. Calcif Tissue Int 41(6):326–331. https://doi.org/10.1007/BF02556671
RGG R, Xia Z, Dunford JE et al (2007) Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci 1117(1):209–257. https://doi.org/10.1196/annals.1402.089
Stopeck AT, Lipton A, Body J-J, Steger GG, Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley d, Viniegra M, Fan M, Jiang Q, Dansey R, Jun S, Braun A (2010) Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 28(35):5132–5139. https://doi.org/10.1200/JCO.2010.29.7101
Author information
Authors and Affiliations
Contributions
Ahmed Elgebaly has full access to all data in the study and takes responsibility for the integrity of presented information and accuracy of the data analysis.
• Study concept and design: Amr Menshawy and Ahmed Elgebaly.
• Internet searching: Amr Menshawy, Omar Mattar, Noha Nasreldin, Ali Abdulkarim, Shirefkasem, Esraa Menshawy, and Salahuddean Mohammed.
• Selection of studies: Omar Mattar, Ali Abdulkarim, and Shirefkasem.
• Data extraction: Amr Menshawy, Omar Mattar, Noha Nasreldin, Ali Abdulkarim, Shirefkasem, Esraa Menshawy, Salahuddean Mohammed, Mohamed Abdel-Maboud, Mohamed Gadelkarim, Gehad Gamal El Ashal, and Ahmed Elgebaly.
• Quality assessment: Amr Menshawy, Mohammed, Mohamed Abdel-Maboud, Mohamed Gadelkarim, Gehad Gamal El Ashal, and Ahmed Elgebaly.
• Data analysis: Ahmed Elgebaly and Gehad Gamal El Ashal.
• Drafting the manuscript: Amr Menshawy, Mohammed, Mohamed Abdel-Maboud, Mohamed Gadelkarim, and Gehad Gamal El Ashal.
• Revision and appraisal of the manuscript: Amr Menshawy, Omar Mattar, Ali Abdulkarim, and Shirefkasem.
• Proofreading the manuscript: Ali Abdulkarim, and Shirefkasem.
• Study monitoring and supervision: Ahmed Elgebaly.
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Menshawy, A., Mattar, O., Abdulkarim, A. et al. Denosumab versus bisphosphonates in patients with advanced cancers-related bone metastasis: systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 26, 1029–1038 (2018). https://doi.org/10.1007/s00520-018-4060-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4060-1