Abstract
Purpose
The study aims to describe mechanisms that contribute to the tendency towards continuing chemotherapy in patients with advanced cancer.
Methods
The study conducted qualitative observations of outpatient clinic visits of 28 patients with advanced cancer (glioblastoma and metastatic colorectal cancer).
Results
We uncovered four mechanisms in daily oncology practice that can contribute to the tendency towards continuing chemotherapy in patients with advanced cancer: (1) “presenting the full therapy sets the standard”—patients seemed to base their justification for continuing chemotherapy on the “standard” therapy with the maximum number of cycles as presented by the physician at the start of the treatment; (2) “focus on standard evaluation moments hampers evaluation of care goals”—whether or not to continue the treatment was mostly only considered at standard evaluation moments; (3) “opening question guides towards focus on symptoms”—most patients gave an update of their physical symptoms in answer to the opening question of “How are you doing?” Physicians consequently discussed how to deal with this at length, which often took up most of the visit; (4) “treatment is perceived as the only option”—patients mostly wanted to continue with chemotherapy because they felt that they had to try every available option the physician offered. Physicians also often seemed to focus on treatment as the only option.
Conclusion
Discussing care goals more regularly with the patient, facilitated for instance by implementing early palliative care, might help counter the mechanisms and enable a more well-considered decision. This could be either stopping or continuing chemotherapy.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
In the past few decades, more and more treatments have become available for cancer patients. If a cure is no longer possible, chemotherapy can be used for life prolongation, symptom control, and maintenance or improvement of quality of life in patients with advanced cancer [1]. Withholding or withdrawing chemotherapy is seen as a realistic option when the effectiveness of treatment is modest and continuing to provide chemotherapy near death is seen as aggressive and inappropriate care. Earle et al., for instance, who defined quality indicators for end-of-life cancer care, had one indicator in which having more than 10 % of the population receiving chemotherapy in the last 14 days of life is considered as poor care [2]. Furthermore, the American Society for Clinical Oncology included stopping end-of-life chemotherapy in its top five practices that could improve patients care (and at the same time reduce costs) at the end of life [3]. However, several studies show that many patients with advanced cancer receive chemotherapy shortly before death [2, 4–6]. In the Netherlands, the tendency towards continuing treatment near death is the subject of public debate. A survey by the Royal Dutch Medical Association (KNMG) in 2012 showed that two thirds of the physicians thought that patients were being overtreated [7].
A qualitative study among Dutch oncologists and nurses about experiences with and attitudes towards the provision of chemotherapy for patients with end-stage cancer found that hope and the wish to prolong life were strong drivers in the tendency to undergo further treatment [8]. Interviews with patients showed that receiving chemotherapy seemed to shift patients’ attention away from the approaching death [9]. While these studies focused on experiences and opinions of healthcare professionals and patients, we have studied how decisions are made in actual practice about whether or not to start second- or third-line chemotherapy for patients with advanced cancer. Our primary aim was to describe how treatment options are discussed and treatment decisions are made in clinical practice. By observing physician-patient encounters and multidisciplinary team meetings, we uncovered mechanisms that can contribute to the tendency towards continuing with chemotherapy in daily advanced cancer care.
Methods
Study design
We conducted a longitudinal qualitative study to get insight in how medical decisions are made during the disease trajectory of advanced cancer patients and therefore observed the outpatient clinic visits of patients at a university hospital.
Study population
Two patient populations facing palliative treatment decisions were included in this study. The first group consisted of patients diagnosed with glioblastoma (GBM), the most common and most malignant type of primary brain tumor in adults, who underwent postoperative combined chemotherapy and radiotherapy [10]. These patients have a poor prognosis and cannot be cured of their disease. The median survival period for these patients is approximately 14 months after diagnosis with current standard care [11]. The second patient population included in the study was a group of patients with metastatic colorectal cancer (mCRC). Patients were included if they were diagnosed with metastatic colorectal cancer (stage IV) and were not eligible for operation. The median survival period for these patients is 24–28 months with current standard care [12], and fewer than 5–8 % of these patients are alive 5 years after the diagnosis [1, 12]. The aims of chemotherapy in both patient populations are to prolong survival, control symptoms, and maintain or improve the quality of life (e.g., relief of pain caused by tumor growth) [1]. Chemotherapy can be effective in prolonging time to disease progression and survival but these benefits must be weighed against treatment toxicity and the effect on the quality of life (e.g. nausea and fatigue) [1].
In both patient groups, as the disease progresses, a decision is often required on whether or not to start a second-line (or third-line) treatment with the disadvantage of burdensome side effects.
Patients diagnosed with GBM were included at the beginning of their adjuvant temozolomide chemotherapy, soon after the end of the postoperative concomitant chemo-irradiation. Patients with mCRC were included at the beginning of the treatment with first-line palliative chemotherapy.
Setting and general treatment regimen
Both patients with GBM and patients with mCRC visit the outpatient clinic once a month to evaluate their current first-line treatment and undergo laboratory examinations. Normally, if blood tests are satisfactory, the next chemotherapy cycle in this first-line treatment will be started. After completion of the first-line treatment (six adjuvant cycles for patients with GBM (12 for patients older than 75 years) and six to eight cycles for patients with mCRC), patients visit the outpatients’ clinic every 3 months or whenever appropriate. All patients will experience tumor recurrence during or after the first-line treatment. Then, a second-line treatment is available. This second-line treatment consisted of six cycles of oral chemotherapy for patients with GBM and of six cycles of intravenous chemotherapy for patients with mCRC. These therapies have been found to have a limited (GBM) or modest (mCRC) effect on life prolongation [13–15]. When the disease progresses again, a third-line treatment—immunotherapy—is available for patients with mCRC. This therapy has not been shown to affect overall survival compared with the best supportive care [16].
During these regimens, the treatment is normally evaluated after every two cycles for patients with GBM and every three cycles for patients with mCRC, using CT or MRI (standard evaluation moments).
Recruitment and inclusion
Patients were recruited in a large university hospital through consecutive sampling. The study with patients with GBM started in May 2010 and patients were included up to December 2012. Recruitment for patients with mCRC started in November 2011 and ended in February 2013. Patients were eligible if they were over the age of 18, spoke and understood Dutch, had been diagnosed with either GBM or mCRC, and had started first-line treatment.
We considered all patients in the two departments who started with first-line therapy during the inclusion period for our study. To identify the patients with GBM, the researcher (LB) attended multidisciplinary team meetings and a twice-weekly briefing where physicians prepared their outpatient clinic visits and discussed patients’ status and treatment plans. To identify patients with mCRC, the researcher attended a weekly briefing where physicians discussed new patients and their treatment plans. She also stayed in regular contact with the physicians and nurses of the participating departments in order to identify new eligible patients.
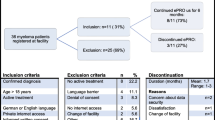
During the inclusion period, we identified 47 patients with GBM and 11 patients with mCRC as potentially eligible for our study (see Fig. 1). Of these, 15 patients with GBM were not actually eligible because the disease was already progressing before the point of inclusion. Furthermore, two patients with GBM were not approached because the physician thought the study would be too burdensome for them. This led to 30 patients with GBM and 11 patients with mCRC who were eligible and were approached for our study. They were handed an information letter by their physician during a visit to the outpatient clinic. Then, 1 week later, the researcher (LB) phoned the patients and explained the study aims and methods to them. Twelve of the patients with GBM and one patient with mCRC declined to participate in the study: five patients were not interested in the study, five patients felt they were too ill to participate, one patient said it was too emotionally demanding because she had problems with her speech, one patient was too stressed about how the disease would develop in future, and one patient did not want the researcher to attend patient-physician conversations.
This resulted in 28 participating patients (68 % of all eligible patients), of whom 18 were diagnosed with GBM and 10 with mCRC. The characteristics of these patients are shown in Table 1.
Data collection
We observed patients at the outpatient clinic visits from the point of inclusion (during the first-line treatment) until patients stopped attending the outpatient clinic because (1) there were no more treatment options available, and patients were referred back to their general practitioner, (2) patients did not want to be treated anymore, or (3) they died.
The visits were audio-recorded and a nonparticipant researcher (LB) observed the visits to make a note of non-verbal communication. LB also had informal conversations with patients and relatives in the waiting room and after the visits when new appointments were made. She also had informal conversations with the physicians before and after visits. In addition, LB was present during staff meetings and multidisciplinary team meetings. Field notes and reflective notes were made of observations on the behavior of patients and staff during and outside visits [17]. Furthermore, LB drew up a summary of each outpatient clinic visit she observed (the content and whether treatment options were discussed). In total, 306 outpatient physician-patient visits were observed in the practices of 22 different oncologists and neurologists. Treatment options were discussed or decisions made during 60 visits. In the remaining 246 visits, only blood tests were discussed and a new cycle of chemotherapy was prescribed.
Data analysis
Data were first analyzed per patient by LB. She used the summaries of the visits and the field notes to make a case summary of each patient focusing on what was discussed and what not, and whether or not treatment options were discussed and if so, when. She also listened to audiotapes of visits in which treatment options were discussed or where results were considered from blood tests (often used for determining the effects of treatment and deciding whether treatment should be continued or not). These case summaries were discussed with one other researcher (HRWP). Based on these case summaries, we identified some patterns in the structure and content of visits and their possible effects on treatment decisions. Examples included physicians describing the standard procedure at the start of the treatment regimen so that patients feel that they have to complete this regimen, and the fact that most of a visit was spent on current symptoms and symptom management, with the care goals hardly being discussed at all. After this first analysis, LB and HRWP looked more in depth at the visits to validate or invalidate these patterns, by listening to the audiotapes of the visits again, and also listening to some other tapes, to see if we were missing other patterns. We selected relevant parts of audiotapes to transcribe verbatim. The separate analyses of the two researchers were compared and discussed. Finally, the findings were discussed within the research group to increase reliability, the main themes were identified, and relevant quotes were chosen to illustrate these themes.
Results
In both patient groups, almost all patients visited the outpatient clinic with partners, children, other family members, or close friends.
We observed that when a treatment regimen was started for a patient, in most cases, it was continued until the last cycle, despite side effects and/or a severe decline in functioning in some patients. Only in some cases did the physician or patient opt to stop with chemotherapy before the end of the general treatment regimen because the burdens outweighed the benefits. The treatment of some patients was stopped before the end of a regimen because the patient was too ill or because blood test results were not good enough. However, in these cases, stopping treatment was not really discussed as an option with the patient; it had already been decided by the physician beforehand and the patient was informed of the decision during the outpatient clinic visit.
We observed four mechanisms in the course and content of the outpatient clinic visits that seem to facilitate this tendency to continue treatment with chemotherapy in the last phase of life of patients with advanced cancer.
Mechanism 1: presenting the full therapy sets the standard
Physicians presented the standard procedure with the number of cycles of chemotherapy they aimed for (six adjuvant cycles for patients with GBM and six to eight cycles for patients with mCRC, accounting for the first-line treatment) at the first outpatient clinic visit after the decision to start first-line treatment. They explained then that if patients experienced toxicity or progression of the disease somewhere during this first-line treatment regimen, second-line and third-line treatment would be available.
Many patients experienced problems or symptoms during the first-line treatment and sometimes, the physician suggested stopping the treatment because of the burden of the therapy. However, most patients wanted to complete the therapy. They seemed to base their justification on the “standard” therapy with the maximum number of cycles as presented by the physician at the start of the treatment. Take the example of this man with mCRC who had severe fatigue and pain at the time of the outpatient clinic visit before starting the third cycle of the second-line treatment regimen:
Physician: “Do you feel okay about having the treatment?”
Patient: “Yes… um, it’s the only option.”
Physician: “You see, I can’t really tell how you feel.”
Patient: “Well, I’ve psyched myself up for it.”
Man with mCRC, age 51–65, cycle 3 of his second-line chemotherapy.
Another example is this woman with GBM with severe fatigue at the time of the visit before starting the tenth cycle of the first-line treatment regimen:
Physician: “I’d say we should try to continue with it. But in the end, you need to say when you think it’s gone far enough.”
Patient: “Yes, but if I stop now then I shouldn’t have started on the second half.”
Physician: “Yes, that often doesn’t feel right. You’re so psyched up to having all 12 cycles.”
Patient: “Quite, I’m not the kind to give up either.”
Woman with GBM, age over 80, cycle 10 of her first-line treatment.
Mechanism 2: the focus on standard evaluation moments hampers the evaluation of care goals
Consideration of whether or not to continue the treatment regimen with a new cycle of chemotherapy, or to start a second-line or third-line treatment regimen, was mostly restricted to the standard evaluation moments using the results from CT or MRI. There was hardly ever any discussion in the visits in between these standard evaluation moments of whether or not to continue the treatment by weighing up the burdens against the benefits and discussing the general goals of care. The prognosis, preferences, and care goals were only occasionally discussed when a patient performed badly in between the standard evaluation moments or when results of blood tests (done before each outpatient clinic visit) were poor. However, when blood tests were inadequate, this generally resulted in the next cycle of chemotherapy being delayed rather than a more general discussion about whether treatment should be continued or not. An example is this conversation with a woman with GBM who had problems with her speech and fatigue at the time of the visit before starting the third cycle of the first-line treatment regimen:
Physician: “The platelet count: we are a little way out of the danger zone, but we’re not high enough to continue with chemotherapy. And then there’s the speech problem which is persisting despite the dexamethasone, but I’m a little worried about the state of the tumour. So I’d really like to do an MRI as well before we go further. This won’t mean any extra delay for you, as we have to wait until the blood is satisfactory again anyway.”
[…].
Physician: “We’ll make a phone appointment with the oncologist next week for the blood results.”
Woman with GBM, age 51–65, delay in third cycle of first-line treatment.
Mechanism 3: opening question guides towards focus on symptoms
Visits at the outpatient clinic were scheduled for 15–20 min. Visits always started with the physician asking the general open question “How are you doing?” This question is probably meant to invite the patient to bring up any subject that they want to discuss. However, practically, all patients gave an update of their physical symptoms or treatment side effects. Physicians consequently paid attention to these symptoms and discussed extensively how to deal with them. This often took up most of the time for the visit. The second part of the visit mostly consisted of medication prescription (not only for the symptoms mentioned, but also for the next cycle of chemotherapy and preventive medication), which took up quite some time. After that, hardly, any time was left for questions from the patient or discussing other things. The more fundamental question of whether they think they are still on the right track and their ideas about the “near future,” including preferences and care goals, were not discussed.
Mechanism 4: treatment is perceived as the only option
When the disease was found to have progressed, patients mostly wanted to start a second-line or third-line treatment regimen. They said they did not want to give up or felt that they had to try every available option the physician offered. An example is this man with mCRC during a visit after a period of remission because of physical complaints:
Patient: “I can’t just say that I want to shake your hand and never see you again; that would be crazy, of course. That’s simply not possible with my problem.”
Physician: “Why not?”
Patient: “Because I’ve got something in me that needs to be treated [...] You’re putting your cards on the table and so I am too. So it is really pie in the sky to say we’re stopping.”
Man with mCRC, age 66–80, period of remission after he had received three cycles of his first-line treatment.
Physicians also often seemed to focus on treatment as the only option, as illustrated in this quote concerning a second operation for a patient with GMB:
Physician: “Now we’ve got two options: chemotherapy without an operation and chemotherapy with an operation. It’s also up to you, you just said you were in favour. I am too, so I reckon that’s what we’re ending up with.”
Man with GBM, age < 35 years, discussing a second operation after progression of the disease.
Discussion
We observed four mechanisms in daily oncology practice that seem to facilitate the tendency to continue chemotherapy in patients with advanced cancer: (1) “presenting the full therapy sets the standard,” (2) “focus on standard evaluation moments hampers the evaluation of care goals,” (3) “opening question guides towards focus on symptoms,” and (4) “treatment is perceived as the only option.” These mechanisms are possibly a result of working in a routine manner at the outpatient clinic, which seems to hamper a more reflective conversation with the patient about care goals. Physicians might not be aware of their routines and the mechanisms.
Furthermore, these mechanisms seem not to be independent. For instance, mechanisms 1 “presenting the full therapy sets the standard” and 2 “focus on standard evaluation moments hampers evaluation of goals” are possibly influenced by mechanism 4 “treatment is seen as the only option.”
The Royal Dutch Medical Association published a report about “appropriate care in the last phase of life,” describing 23 mechanisms that contribute to continuing with treatment on patient/physician level, institutional level, and national level [18]. On the institutional level, they mention that treatment guidelines are focussed on continuing treatment. This mechanism resonates with mechanisms 1 and 2 we found on patient/physician level. “Treatment is seen as the only option” is also described by them, both on patient/physician level, as on national level. They conclude, among other things, that this mechanism is dominant in the Dutch society. They recommend that more room for accepting death and dying should be created nationally, and propose setting up national campaigns and stimulating advance care planning as most important interventions to promote appropriate care at the end of life.
Strengths and weaknesses of the study
The longitudinal design with observational data combined with informal conversation data gave us an in-depth insight into daily oncology practice and made it possible to uncover the mechanisms. However, this is the daily practice of two departments in one university hospital in the Netherlands, and the results might not be applicable in other settings. Furthermore, the observations were done by one researcher, which might have created researcher bias. To counter this bias, the researcher had regular meetings with the research team during the data collection period and discussed her findings with the team. Furthermore, the analyses were performed by two researchers and discussed regularly with the research team [19].
Status quo
The observed mechanisms seem to have been present in daily practice for years, since they resonate well with results from studies performed in the 1990s, for instance, a study on false optimism about recovery from small-cell lung cancer [20]. The et al. observed that outpatient clinic visits were mainly focused on which treatment options were available and were restricted to issues such as planning new chemotherapy, side effects, and test results. The longer term perspective and general care goals were ignored in the discussions. Furthermore, Charles et al. [21] found back in the 1990s that women with early-stage breast cancer thought that “doing nothing” was not an option. The authors stated that this is partly an effect of the way options were communicated to the women, i.e., as “doing something” versus doing nothing. Most women did not perceive this as a meaningful choice. Koedoot et al. [22] found that in their study, half of the patients with advanced cancer were not told by the physician that stopping treatment was an option, the physician only mentioned that doing nothing was also an option to a quarter of patients while the option of doing nothing was explained more extensively in the remaining quarter of the cases. They concluded that if patients are not told about all the options in detail, they are not equipped to make a well-informed decision.
It is striking that daily oncology practice has apparently not changed in the last couple of decades, despite the emphasis that has been put on the importance of shared decision-making and advance care planning in the past decade.
In shared decision-making, the physician and patient share information and treatment preferences, and both aim to reach mutual agreement about what steps to take in disease management [23, 24]. An important step involves the physician outlining all treatment options, including the option of doing nothing, and explaining that there is no best option, thereby “creating awareness of equipoise” [25].
Advance care planning implies timely and regular involvement of patients and their proxies in decision-making with respect to the future goals of treatment and end-of-life care [26]. Apparently, shared decision-making and advance care planning are still not integrated in daily advanced cancer care, at least not in the daily practice we observed.
How to achieve change?
Allowing time for reflection during outpatient clinic visits might act as a counter to routine behavior. This could be done by regularly discussing and re-evaluating the goals of care with the patient. Mack et al. found that patients who had end-of-life discussions with their physician before the last 30 days of life were less likely to receive chemotherapy in the last 14 days of life [4]. Greer et al. found that patients who received “early palliative care” had less chance of receiving chemotherapy in the last 60 days of life [27]. Early palliative care included having patients meet with a member of a palliative care team shortly after diagnosis and at least monthly thereafter in the outpatient clinic until death. The guidelines for consultation with the palliative care team included five topics (understanding the illness, symptom management, decision-making, coping, and planning and referral) [28]. The discussion of longer term perspectives and care goals is facilitated by structuring the outpatient clinic visits around topics. This structuring and associated broadening of the visits might help counter the mechanisms we observed in our study that seem to facilitate continuing chemotherapy.
Specifically regarding the last mechanism (“treatment is perceived as the only option”), Quill et al. stress that when patients say they want “everything,” a physician should make efforts to understand what this means to the patient [29]. They describe different treatment philosophies that may underlie doing everything, ranging from “everything that has any possible potential to prolong life even a small amount, regardless of its effect on the patient ‘s suffering” to “everything that might provide maximum relief of suffering, even if it might unintentionally shorten life.” They also provide examples of useful questions to ask patients in different situations [29]. They stated that you can only propose the possible treatment options that are consistent with the patient’s values and priorities when you know what the patient really wants.
Continuing chemotherapy can be a well-considered decision, but from our study, it seems that mechanisms in daily practice hamper a well-considered decision and individualistic approach, instead fostering a standard approach and treatment plan.
References
Simmonds PC (2000) Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ 321(7260):531–535
Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ (2008) Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol 26(23):3860–3866
Schnipper LE, Smith TJ, Raghavan D, Blayney DW, Ganz PA, Mulvey TM, Wollins DS (2012) American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol 30(14):1715–1724
Mack JW, Weeks JC, Wright AA, Block SD, Prigerson HG (2010) End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol 28(7):1203–1208
Zietemann V, Duell T (2010) Every-day clinical practice in patients with advanced non-small-cell lung cancer. Lung Cancer 68(2):273–277
Frigeri M, De DS, Castillo-Fernandez O, Feuerlein K, Neuenschwander H, Saletti P (2013) Chemotherapy in patients with advanced pancreatic cancer: too close to death? Support Care Cancer 21(1):157–163
Visser J (2012) De arts staat in de behandelmodus. Medisch Contact 67(22):1326–1329
Buiting HM, Rurup ML, Wijsbek H, van Zuylen L, den Hartogh G (2011) Understanding provision of chemotherapy to patients with end stage cancer: qualitative interview study. BMJ 342:d1933
Buiting HM, Terpstra W, Dalhuisen F, Gunnink-Boonstra N, Sonke GS, den Hartogh G (2013) The facilitating role of chemotherapy in the palliative phase of cancer: qualitative interviews with advanced cancer patients. PLoS One 8(11):e77959
Taphoorn MJ, Stupp R, Coens C, Osoba D, Kortmann R, van den Bent MJ, Mason W, Mirimanoff RO, Baumert BG, Eisenhauer E, Forsyth P, Bottomley A (2005) Health-related quality of life in patients with glioblastoma: a randomised controlled trial. Lancet Oncol 6(12):937–944
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Chu E (2012) An update on the current and emerging targeted agents in metastatic colorectal cancer. Clin Colorectal Cancer 11(1):1–13
Hau P, Baumgart U, Pfeifer K, Bock A, Jauch T, Dietrich J, Fabel K, Grauer O, Wismeth C, Klinkhammer-Schalke M, Allgäuer M, Schuierer G, Koch H, Schlaier J, Ulrich W, Brawanski A, Bogdahn U, Steinbrecher A (2003) Salvage therapy in patients with glioblastoma: is there any benefit? Cancer 98(12):2678–2686
Kappelle AC, Postma TJ, Taphoorn MJ, Groeneveld GJ, van den Bent MJ, van Groeningen CJ, Zonnenberg BA, Sneeuw KC, Heimans JJ (2001) PCV chemotherapy for recurrent glioblastoma multiforme. Neurology 56(1):118–120
Oostendorp Stalmeier PF LJ, Pasker-de Jong PC, van der Graaf WT, Ottevanger PB (2010) Systematic review of benefits and risks of second-line irinotecan monotherapy for advanced colorectal cancer. Anti-Cancer Drugs 21(8):749–758
Cutsem V, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25(13):1658–1664
Mays N, Pope C (1995) Observational methods in health care settings. BMJ 311:182–184
Royal Dutch Medical Association (2015). [Niet alles wat kan, hoeft.] Appropriate care in the last phase of life. Report of the steering committee Appropriate care at the end of life. Utrecht
Mays N, Pope C (1995) Rigour and qualitative research. BMJ 311:109–112
The AM, Hak T, Koëter G, van Der Wal G (2000) Collusion in doctor-patient communication about imminent death: an ethnographic study. BMJ 321(7273):1376–1381
Charles C, Redko C, Whelan T, Gafni A, Reyno L (1998) Doing nothing is no choice: lay constructions of treatment decision-making among women with early-stage breast cancer. Sociology of Health & Illness 20:71–95
Koedoot CG, Oort FJ, de Haan RJ, Bakker PJ, de Graeff A, de Haes JC (2004) The content and amount of information given by medical oncologists when telling patients with advanced cancer what their treatment options are palliative chemotherapy and watchful-waiting. Eur J Cancer 40(2):225–235
Charles C, Gafni A, Whelan T (1997) Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 44(5):681–692
Brock DW (1991) The ideal of shared decision making between physicians and patients. Kennedy Inst Eth J 1:28–47
Stiggelbout AM, Van der Weijden T, De Wit MPT, Frosch D, Légaré F, Montori VM, Trevena L, Elwyn G (2012) Shared decision making: really putting patients at the centre of healthcare. BMJ 344:28–31
Singer PA, Robertson G, Roy DJ (1996) Bioethics for clinicians: 6. Advance care planning. CMAJ 155:1689–1692
Greer JA, Pirl WF, Jackson VA, Muzikansky A, Lennes IT, Heist RS, Gallagher ER, Temel JS (2012) Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol 30(4):394–400
Jacobsen J, Jackson V, Dahlin C, Greer J, Perez-Cruz P, Billings JA, Pirl W, Temel J (2011) Components of early outpatient palliative care consultation in patients with metastatic nonsmall cell lung cancer. J Palliat Med 14(4):459–464
Quill TE, Arnold R, Back AL (2009) Discussing treatment preferences with patients who want “everything”. Ann Intern Med 151(5):345–349
Acknowledgments
We thank all the patients and physicians who shared their experiences and opinions with us. Their contribution to the research is invaluable.
Contributors
BDO-P and HRWP had the initial idea for this study and wrote the research proposal. LB did the observations. LB and HRWP did the coding and analyses, which was discussed with BDO-P and GAMW. LB wrote the first draft. HRWP, BDO-P, and GAMW commented on and contributed to the final draft. BDO-P is a guarantor. All contributors had access to all the data and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study was funded by the Innovational Research Incentives Scheme VICI 2008 from the Netherlands Organisation for Scientific Research (NWO).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study protocol was approved by the Medical Ethics Committee of the VU University medical center. The participating departments gave their approval for the research to be carried out. Informed consent was obtained from all the participating patients and physicians.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Brom, L., Onwuteaka-Philipsen, B.D., Widdershoven, G.A. et al. Mechanisms that contribute to the tendency to continue chemotherapy in patients with advanced cancer. Qualitative observations in the clinical setting. Support Care Cancer 24, 1317–1325 (2016). https://doi.org/10.1007/s00520-015-2910-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2910-7