Abstract
Purpose
Subcutaneous APF530 provides controlled sustained release of granisetron to prevent acute (0–24 h) and delayed (24–120 h) chemotherapy-induced nausea and vomiting (CINV). This randomized, double-blind phase 3 trial compared APF530 and palonosetron in preventing acute and delayed CINV after moderately (MEC) or highly emetogenic chemotherapy (HEC).
Methods
Patients receiving single-day MEC or HEC received single-dose APF530 250 or 500 mg subcutaneously (SC) (granisetron 5 or 10 mg) or intravenous palonosetron 0.25 mg. Primary objectives were to establish APF530 noninferiority to palonosetron for preventing acute CINV following MEC or HEC and delayed CINV following MEC and to determine APF530 superiority to palonosetron for preventing delayed CINV following HEC. The primary efficacy end point was complete response (CR [using CI difference for APF530 − palonosetron]). A lower confidence bound greater than −15 % indicated noninferiority.
Results
In the modified intent-to-treat population (MEC = 634; HEC = 707), both APF530 doses were noninferior to palonosetron in preventing acute CINV after MEC (CRs 74.8 % [−9.8, 9.3] and 76.9 % [−7.5, 11.4], respectively, vs. 75.0 % palonosetron) and after HEC (CRs 77.7 % [−11.5, 5.5] and 81.3 % [-7.7, 8.7], respectively, vs. 80.7 % palonosetron). APF530 500 mg was noninferior to palonosetron in preventing delayed CINV after MEC (CR 58.5 % [−9.5, 12.1] vs. 57.2 % palonosetron) but not superior in preventing delayed CINV after HEC. Adverse events were generally mild and unrelated to treatment, the most common (excluding injection-site reactions) being constipation.
Conclusions
A single subcutaneous APF530 injection offers a convenient alternative to palonosetron for preventing acute and delayed CINV after MEC or HEC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Supportive care is essential for patients with cancer receiving chemotherapy. Advances in understanding the physiology of emesis led to the development of receptor-targeted antiemetics (5-hydroxytryptamine 3 [5-HT3] antagonists, neurokinin-1 [NK-1] antagonists) and resulted in evidence-based guidelines for prevention of chemotherapy-induced nausea and vomiting (CINV) for acute (within 24 h of chemotherapy administration) and delayed (24–120 h after chemotherapy administration) phases [1–3].
An important risk factor for CINV is the emetogenicity of the chemotherapy regimen, first classified by the Hesketh algorithm [4]. Clinical practice guidelines [1–3] recommend a combination of a 5-HT3 antagonist, dexamethasone, and an NK-1 antagonist to prevent CINV after administration of highly emetogenic chemotherapy (HEC). For moderately emetogenic chemotherapy (MEC), guidelines differ somewhat depending on the chemotherapy regimen but generally recommend a 5-HT3 antagonist plus dexamethasone (plus a NK-1 antagonist for select patients).
Nevertheless, CINV prevention (especially delayed CINV) remains a significant problem and is considerably underestimated by physicians and nurses [5]. Consequently, strategies to improve CINV prevention (especially delayed) are needed. First-generation 5-HT3 antagonists (ondansetron, granisetron, dolasetron [oral]) are administered daily because of their short half-lives, usually on day 1 of chemotherapy only [1, 3, 6]. They may be administered on days 2 and 3 to control delayed CINV, but their efficacy and cost-effectiveness in this setting are questionable [7]. Palonosetron, a second-generation 5-HT3 antagonist with a longer half-life (∼40 h), can be administered on day 1 of chemotherapy only [1, 3, 8–12]. Several hypotheses, based on preclinical studies, have been proposed for its mechanism of action, including 5-HT3 receptor internalization and prolonged inhibition of 5-HT3 receptor function that continues after palonosetron has dissociated from the cell surface [13, 14].
APF530 is a new, subcutaneously (SC) administered polymeric formulation of granisetron that was developed to provide slow, controlled, and sustained release of granisetron to prevent both acute and delayed CINV associated with MEC and HEC [15]. APF530 comprises 2 % granisetron and a polymer vehicle of tri(ethylene glycol) poly(orthoester) (TEG-POE) that undergoes controlled hydrolysis, resulting in slow, controlled, and sustained drug release. The novel biodegradable polymeric excipient is hydrolyzed in vivo, generating nontoxic biodegradable metabolites. This Biochronomer™ drug delivery system (Heron Therapeutics, Inc., Redwood City, CA) allows therapeutic levels of granisetron to be maintained for >5 days with a single subcutaneous injection. In clinical studies in patients undergoing chemotherapy, single-dose APF530 (5–15 mg granisetron) administered SC in the abdomen provided circulating levels of granisetron within 30 min, a maximum plasma concentration at ∼24 h, and sustained therapeutic levels for >120 h.
In this phase 3 noninferiority trial, the clinical efficacy of APF530 250 and 500 mg SC (containing granisetron 5 and 10 mg, respectively) was compared with that of the approved dose of palonosetron (0.25 mg intravenously [IV]) for prevention of acute and delayed CINV following single-day administration of MEC or HEC in patients with cancer.
Methods
Patients
Eligible patients were men or women ≥18 years old with histologically or cytologically confirmed malignancy and scheduled to receive single-day MEC or HEC according to then-applicable Hesketh criteria [4, 16]. All patients provided written informed consent. At least 7 days before study drug administration, patients discontinued antiemetics and systemic corticosteroids (including dexamethasone). Planned chemotherapy was to be ≤4 h (excluding monoclonal antibodies). Patients could be chemotherapy naïve or nonnaïve and had to have Eastern Cooperative Oncology Group (ECOG) performance status ≤2.
Key exclusion criteria included gastrointestinal cancers or intestinal obstruction, head and neck cancers, or primary brain tumors or metastases with the potential to affect nausea and vomiting centers in the brain; receipt of chemotherapy during the 7 days before study drug administration; no vomiting or more than mild nausea within 24 h before study drug administration; a QTc interval >500 ms or a >60 ms change from baseline; or other cardiac abnormality predisposing to significant arrhythmia.
Study design and treatments
This prospective, multicenter, randomized, double-blind, double-dummy, parallel-group phase 3 trial (clinicaltrials.gov identifier: NCT00343460) was approved by the Institutional Review Board or Independent Ethics Committee at each center and conducted according to the Declaration of Helsinki.
Enrolled patients received single-day MEC or HEC, defined by the Hesketh algorithm [4, 16]. Chemotherapeutic agents causing emesis in <10 % of patients were defined as level 1 agents, 10–30 % as level 2, 30–60 % as level 3, 60–90 % as level 4, and >90 % as level 5. For combination regimens, the most emetogenic agent was identified (as level 1–5), then the relative contribution of each additional agent was added as follows: a level 1 agent did not contribute to emetogenicity; one or more level 2 agents increased emetogenicity by one level greater than the most emetogenic agent; and one or more level 3 or 4 agents increased emetogenicity by one level per agent more than the most emetogenic agent.
Patients were stratified according to their chemotherapy emetogenicity (MEC or HEC) and randomized 1:1:1 to receive APF530 250 mg SC (granisetron 5 mg) plus placebo IV; APF530 500 mg SC (granisetron 10 mg) plus placebo IV; or palonosetron IV 0.25 mg plus placebo SC (Fig. 1) prior to chemotherapy. Isotonic saline was used for placebo SC injections and intravenous infusions. After cycle 1, all patients were invited to continue in the study. If they consented, they were rerandomized to maintain blinding, but only patients who received palonosetron in cycle 1 were actually randomized 1:1 to receive APF530 250 or 500 mg SC for ≤3 subsequent cycles.
On day 1, study drug was administered 30–60 min before single-day MEC or HEC. All subcutaneous injections were administered abdominally; a local anesthetic could be administered beforehand. Protocol-specified doses of dexamethasone were administered 30–90 min before chemotherapy (8 mg IV for MEC, 20 mg IV for HEC). On days 2–4, oral dexamethasone 8 mg twice daily was prescribed to patients receiving HEC. Rescue medications were allowed as needed. APF530 SC was administered in ≤4 treatment cycles separated by 7 to 28 days (±3); palonosetron IV was discontinued after cycle 1.
Objectives and efficacy evaluations
The primary objectives were to establish, during cycle 1, noninferiority of APF530 SC to palonosetron IV in preventing acute CINV (0–24 h) following MEC or HEC administration, noninferiority of APF530 SC to palonosetron IV in preventing delayed CINV (24–120 h) following MEC administration, and superiority of APF530 SC to palonosetron IV in preventing delayed CINV (24–120 h) following HEC administration. Secondary objectives included evaluation of efficacy over the entire (0–120 h) period during cycle 1 and evaluation of safety and tolerability of APF530 SC.
The primary efficacy end point was the percentage of patients achieving a complete response (CR; no emetic episodes and no use of rescue medications) during the acute (0–24 h) and delayed (24–120 h) phases after chemotherapy cycle 1. Secondary end points included safety and percentage of patients with CR over the entire (0–120 h) period during cycle 1, both of which are reported here. Other secondary end points (not reported) included percentages of patients with complete control (CC; CR with no more than mild nausea) and total response (TR; CR with no nausea) during the acute and delayed phases in cycle 1; assessment of APF530 SC pharmacokinetics in a subset of patients receiving MEC or HEC during cycle 1; and measurement of cardiac safety using electrocardiographic monitoring during cycle 1. Efficacy measures were determined from diaries, in which patients recorded emetic episodes, rescue medication, and severity of nausea for each 24-h period after chemotherapy.
At the time that this study was conducted, chemotherapy regimens containing cyclophosphamide plus anthracyclines were classified as moderately emetogenic [4, 16]. However, in 2011, the American Society of Clinical Oncology reclassified cyclophosphamide plus anthracycline regimens as highly emetogenic in its antiemesis guidelines [2]. Consequently, at the request of the US Food and Drug Administration (FDA), a post hoc analysis of efficacy end points was undertaken with these combination regimens reclassified as HEC.
Safety evaluations
Adverse events (AEs; based on standard toxicity criteria) and serious AEs (SAEs) were evaluated during each treatment cycle, including type, duration, severity (mild, moderate, severe), and investigator’s opinion in relation to study drug. Physical examinations, vital signs, and clinical laboratory parameters were also assessed.
Statistical analysis
The planned sample size was 1,404 patients, 669 in the MEC stratum (n = 223 per treatment group for cycle 1) and 735 in the HEC stratum (n = 245 per treatment group in cycle 1). For the MEC stratum, sample size was based on the assumption of a CR of 65 % in any treatment group and a difference of at least 15 % in CR. For the HEC stratum, sample size was based on the assumption of a CR of 50 % in any treatment group and a difference of at least 15 % in CR.
In the primary efficacy analysis, noninferiority was tested for six comparisons: two APF530 SC doses for both acute and delayed CR in patients receiving MEC and for acute CR in patients receiving HEC. Superiority was tested between the APF530 SC doses and palonosetron for delayed CR in patients receiving HEC. To adjust for the effect of multiple comparisons, analyses used Hochberg’s sharper Bonferroni procedure, a stepwise multistage approach in which the level of confidence is increased at each successive stage [17]. At each stage, noninferiority was demonstrated when the lower bound of the confidence interval (CI) for CR for the difference between APF530 and palonosetron (APF530 − palonosetron) was greater than −15 %, and superiority was demonstrated when the lower bound of the CI was greater than 0 %; the noninferiority margin of 15 % was selected as it was the same margin used in palonosetron noninferiority clinical trials [8–10]. Treatment comparisons were based on Fisher’s exact test.
Efficacy analyses were performed separately for MEC and HEC strata and based on a modified intent-to-treat (mITT) population, comprising all randomized patients who received study drug and had postbaseline efficacy data. The safety population comprised all patients who were randomized and received study drug.
Quantitative variables were summarized by sample size, mean, median, standard deviation (SD), minimum, and maximum. Qualitative variables were summarized by patient number and percentage. Unless otherwise indicated, statistical significance was reached if the two-sided p value was <0.05.
Results
Patient characteristics
The study was conducted between June 2006 and August 2008 at 103 centers in the USA, India, and Poland. Of 1,428 randomized patients, 33 did not receive study drug and were excluded, leaving 1,395 patients evaluable for efficacy and safety (n = 653, MEC; n = 742, HEC; defined by Hesketh criteria) [4]. The disposition of patients by study drug (APF530 250 or 500 mg SC, palonosetron 0.25 mg IV) and by MEC and HEC for cycle 1 is shown in Fig. 2. In total, 639 patients (97.9 %) receiving MEC and 727 patients (98.0 %) receiving HEC completed cycle 1. The most common reasons for discontinuation were loss to follow-up (n = 10, MEC; n = 3, HEC), withdrawal of consent (n = 2, MEC; n = 2, HEC), and death (n = 1, MEC; n = 3, HEC). Of 1,341 patients who completed cycle 1, 1,043 (76.4 %) were rerandomized to APF530 250 or 500 mg SC prior to cycle 2. The most common MEC regimen was based on cyclophosphamide plus an anthracycline (56.0 % of patients), and the most common HEC regimen was based on carboplatin plus a taxane (40.3 %) (Table 1).
Patient disposition during cycle 1. According to Hesketh criteria [4] (superscript a). Safety population (superscript b)
Baseline demographics and clinical characteristics (mITT population) were similar across all treatment arms and across both MEC and HEC strata (Table 2). Most patients were women (62.8–87.7 % across emetogenicity strata and treatment arms), and mean age ranged from 54.8 to 58.1 years. The most common tumor types were breast cancer (63.3–69.5 %) in the MEC stratum and lung (25.4–32.8 %) and breast cancers (25.4–27.6 %) in the HEC stratum. Overall, 319 patients (48.9 %) receiving MEC and 426 patients (57.4 %) receiving HEC had received prior chemotherapy.
Primary efficacy analysis
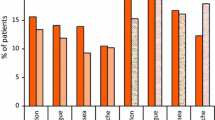
Results presented here are for the original prespecified analysis, in which chemotherapy emetogenicity was determined using Hesketh criteria [4]. In cycle 1, both APF530 250 and 500 mg SC were noninferior to palonosetron, as assessed by CR, in the control of acute CINV after MEC, with CRs (97.5 % CI difference vs. palonosetron) of 74.8 % (−9.8, 9.3) and 76.9 % (−7.5, 11.4), respectively, versus 75.0 % for palonosetron (Fig. 3a). The result was similar for patients receiving HEC, with acute CRs (98.33 % CI difference vs. palonosetron) of 77.7 % (−12.1, 6.1) and 81.3 % (−8.2, 9.3) for APF530 250 and 500 mg SC, respectively, versus 80.7 % for palonosetron (Fig. 3b).
Complete response during acute and delayed CINV phases with APF530 250 and 500 mg SC and palonosetron (PALO) 0.25 mg IV after administration of a MEC and b HEC (according to Hesketh criteria [4]) in cycle 1 (modified intent-to-treat population)
APF530 500 mg SC also was noninferior to palonosetron in preventing delayed CINV after MEC, with a CR of 58.5 % (−9.5, 12.1) versus 57.2 % for palonosetron (Fig. 3a). Superiority of APF530 250 or 500 mg SC versus palonosetron 0.25 mg IV in preventing delayed CINV after HEC in cycle 1 was not achieved, although CR rates were similar for APF530 500 mg SC and palonosetron 0.25 mg IV (CRs 67.1 vs. 64.3 % [−5.8, 11.3], respectively) (Fig. 3b).
Secondary efficacy analyses
A secondary efficacy objective was evaluation of APF530 SC efficacy over the entire (0–120 h) period during cycle 1. After administration of MEC, overall CRs (95 % CI difference vs. palonosetron) with APF530 250 and 500 mg SC were 48.6 % (−2.9, 6.2) and 53.8 % (−7.8, 11.4), respectively, versus 51.9 % for palonosetron 0.25 mg IV.
After administration of HEC, CRs (95 % CI difference vs. palonosetron) with APF530 250 and 500 mg SC were 57.6 % (−11.8, 6.1) and 63.3 % (−5.9, 11.6), respectively, versus 60.5 % for palonosetron 0.25 mg IV over the entire treatment period (0–120 h).
Post hoc analysis of efficacy
In a post hoc analysis, patients receiving chemotherapy regimens whose antiemetic risk had been revised according to updated antiemetic practice guidelines, notably cyclophosphamide plus anthracyclines (reclassified from MEC to HEC) and carboplatin-based regimens (reclassified from HEC to MEC), were reclassified at the request of the FDA [2]. The results of this reanalysis showed no notable statistical or clinical differences in response rates between APF530 and palonosetron.
Safety and tolerability
The safety population comprised all patients (MEC and HEC) who were randomized and received study drug (n = 1,395). Safety analyses were conducted on the combined MEC and HEC populations and are presented for cycle 1 only (Table 3). In cycle 1, 464 patients received APF530 250 mg SC, 468 received APF530 500 mg SC, and 463 received palonosetron 0.25 mg IV. Overall, AEs were generally mild and most were considered unrelated to treatment. Excluding injection-site reactions (ISRs), the most common AE was constipation (occurring in 13.4–15.6 % of patients across all groups), followed by fatigue (in 11.9–14.1 % of patients). Excluding ISRs, the most frequent treatment-related AEs were mild constipation (in 2.6–3.2 % of patients across all groups) and mild headache (in 0.6–2.4 % of patients). Excluding ISRs, two patients had treatment-related AEs that led to study discontinuation in cycle 1: moderate dyspepsia in the APF530 250 mg SC group and mild drug hypersensitivity in the APF530 500 mg SC group. Severe treatment-related AEs occurred in 15 patients, including 4 (0.9 %) in the APF530 250 mg SC group, 6 (1.3 %) in the APF530 500 mg SC group, and 5 (1.1 %) in the palonosetron 0.25 mg IV group. There were no significant differences between treatment groups in percentages of patients who had AEs, percentages of patients who discontinued because of a treatment-related AE, or severity of treatment-related AEs.
Excluding ISRs, only one patient (0.2 %) in the APF530 250 mg SC group experienced a serious treatment-related AE (pulmonary embolism), 16 days after receiving study medication; the event was considered possibly related to treatment, and the patient was withdrawn from the study. Eleven patients died in cycle 1: seven receiving APF530 250 mg SC, two receiving APF530 500 mg SC, and two receiving palonosetron 0.25 mg IV; but none of the deaths was considered to be related to treatment.
ISRs occurred in all treatment groups (Table 3). The most common ISR during cycle 1 was bruising (in 9.1–19.9 % of patients across all treatment groups), followed by erythema (3.5–10.9 % of patients) and nodules (0.6–10.7 % of patients). Treatment-related ISRs were more frequent in the APF530 groups than in the palonosetron group, but were generally mild and resolved over time; the most commonly reported treatment-related ISR was bruising. There were no severe ISRs in cycle 1, and no patients discontinued therapy as a result of an ISR.
There were no notable changes in physical examination or clinical laboratory parameters.
Discussion
In this study, one of the largest prospective, randomized trials conducted in CINV to date, APF530 250 and 500 mg SC (containing granisetron 5 and 10 mg, respectively) demonstrated noninferiority to palonosetron 0.25 mg IV in controlling acute CINV in patients who received MEC or HEC, as determined by CR. APF530 500 mg SC was also noninferior to palonosetron in preventing delayed CINV in patients receiving MEC. Furthermore, APF530 SC demonstrated antiemetic efficacy over the entire 120-h period after MEC or HEC comparable to that of palonosetron. These results were achieved in a patient population with a variety of cancer types and receiving a range of chemotherapy regimens. The safety profiles of both APF530 SC doses were similar to that of palonosetron and consistent with previous clinical experience with granisetron in the CINV setting [18, 19]. ISRs occurred across all treatment groups, but were generally mild and resolved by the end of the study. Thus, APF530 SC appears to add an option to the existing formulations of granisetron (IV, oral, and transdermal), providing sustained release of the drug for prevention of both acute and delayed CINV in patients receiving MEC or HEC.
Limitations of this study include the fact that, at the time the study was designed, palonosetron did not have an established benefit in preventing delayed CINV in patients receiving HEC, so a noninferiority margin could not be set. In addition, some criteria used to classify regimens as MEC or HEC have been updated since the study was conducted. Notably, regimens containing cyclophosphamide plus anthracyclines classified as MEC in this study are now considered to be HEC, and carboplatin-based regimens classified as HEC in this study are now considered to be MEC [1–4]. Assigning emetic risk of chemotherapy agents can be controversial, and this is illustrated in the current study. Few chemotherapy agents have been prospectively evaluated to determine their true emetic rate when antiemetics are not given. All rating scales are based on expert consensus, rather than on true high-quality evidence. The Hesketh classification was a diligent effort to try to place combination chemotherapy, rather than just single agents, into a useful system. As with all emetic rating systems, it has limitations. Paradoxically, as seen in the results of the current study, emetic control for all randomized arms was somewhat higher in patients receiving HEC than it was for those receiving MEC, according to the Hesketh system. Limitations of current criteria used in treatment guidelines can be seen in the fact that groups have reclassified emetic risk even of older chemotherapy agents (such as cyclophosphamide plus anthracyclines) more than a decade after first listing these agents [1–3]. It was appropriate in this study to give results by older and newer risk criteria, as requested by the FDA. However, it is notable that a post hoc analysis reclassifying patients according to the new antiemetic risk criteria showed no notable statistical or clinical differences in efficacy measures between APF530 and palonosetron; so, it did not alter the conclusions. Moreover, antiemetic guidelines updated after this study was initiated [1–3] now recommend the use of aprepitant as CINV prophylaxis in patients receiving HEC.
To date, several large randomized trials have demonstrated that palonosetron is at least as effective as ondansetron [8, 10], dolasetron [9], and granisetron [11] in preventing CINV in patients receiving MEC or HEC. However, in the current trial, a new subcutaneous formulation of granisetron was noninferior to palonosetron (an agent broadly recommended as the preferred 5-HT3 antagonist in treatment guidelines [1–3]), in the control of acute CINV in patients receiving MEC or HEC and delayed CINV in patients receiving MEC. It has been postulated that the superiority of palonosetron over first-generation agents is in part due to its unique characteristics, including cooperative interactions with substance P and the NK-1 receptor pathway [20]. Our findings of noninferiority of this granisetron formulation to palonosetron appear to question this hypothesis. From the results of this study, it appears that both APF530 and palonosetron provide good antiemetic control in the settings tested, and oral palonosetron was recently reported to be noninferior to palonosetron IV in preventing acute CINV in patients receiving MEC [21]. The convenience of a single SC injection of APF530 provides an alternative option to palonosetron IV for the prevention of CINV. Post hoc analyses may provide further insights into the efficacy of APF530 SC and the effects of sex, age, type of chemotherapy regimen administered, and prior therapies. Further studies may investigate the potential for APF530 in other clinical situations where sustained antiemetic activity is desired, such as during multiday chemotherapy, in patients receiving radiation therapy, in patients who are unable to tolerate oral antiemetics, in combination with aprepitant and other NK-1 antagonists, and in the postoperative setting.
References
NCCN Clinical Practice Guidelines in Oncology: Antiemesis—v1.2014. http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf
Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM, Freundlich B, Morrow G, Rao KV, Schwartz RN, Lyman GH (2011) Antiemetics: American society of clinical oncology clinical practice guideline update. J Clin Oncol 29:4189–4198
Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21(suppl 5):v232–v243
Hesketh PJ, Kris MG, Grunberg SM, Beck T, Hainsworth JD, Harker G, Aapro MS, Gandara D, Lindley CM (1997) Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 15:103–109
Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, Daniele B, De Pourville G, Rubenstein EB, Daugaard G (2004) Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 100:2261–2268
Roila F, Warr D, Clark-Snow RA, Tonato M, Gralla RJ, Einhorn LH, Herrstedt J (2005) Delayed emesis: moderately emetogenic chemotherapy. Support Care Cancer 13:104–108
Geling O, Eichler HG (2005) Should 5-hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol 23:1289–1294
Aapro MS, Grunberg SM, Manikhas GM, Olivares G, Suarez T, Tjulandin SA, Bertoli LF, Yunus F, Morrica B, Lordick F, Macciocchi A (2006) A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol 17:1441–1449
Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, Macciocchi A, Grunberg S (2003) Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer 98:2473–2482
Gralla R, Lichinitser M, Van DV, Sleeboom H, Mezger J, Peschel C, Tonini G, Labianca R, Macciocchi A, Aapro M (2003) Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 14:1570–1577
Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, Inoue K, Kitagawa C, Ogura T, Mitsuhashi S (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10:115–124
Roila F, Warr D, Aapro M, Clark-Snow RA, Einhorn L, Gralla RJ, Herrstedt J, Saito M, Tonato M (2011) Delayed emesis: moderately emetogenic chemotherapy (single-day chemotherapy regimens only). Support Care Cancer 19(Suppl 1):S57–S62
Rojas C, Thomas AG, Alt J, Stathis M, Zhang J, Rubenstein EB, Sebastiani S, Cantoreggi S, Slusher BS (2010) Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol 626:193–199
Rojas C, Raje M, Tsukamoto T, Slusher BS (2013) Molecular mechanisms of 5-HT(3) and NK(1) receptor antagonists in prevention of emesis. Eur J Pharmacol 722:26–37
Heller J, Barr J (2005) Biochronomer technology. Expert Opin Drug Deliv 2:169–183
Hesketh PJ (1999) Defining the emetogenicity of cancer chemotherapy regimens: relevance to clinical practice. Oncologist 4:191–196
Hochberg J (1988) A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800–802
(2011) Kytril (granisetron hydrochloride) injection. Prescribing information. South San Francisco, CA: Genentech, Inc
(2010) Kytril (granisetron hydrochloride) tablets oral solution. Prescribing information. Roche Pharmaceuticals, Nutley, NJ
Rojas C, Li Y, Zhang J, Stathis M, Alt J, Thomas AG, Cantoreggi S, Sebastiani S, Pietra C, Slusher BS (2010) The antiemetic 5-HT3 receptor antagonist palonosetron inhibits substance P-mediated responses in vitro and in vivo. J Pharmacol Exp Ther 335:362–368
Boccia R, Grunberg S, Franco-Gonzales E, Rubenstein E, Voisin D (2013) Efficacy of oral palonosetron compared to intravenous palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: a phase 3 trial. Support Care Cancer 21:1453–1460
Acknowledgments
The authors would like to thank the many investigators and their clinical staff who made this study possible and thank the employees at Heron Therapeutics, Inc. (formerly A.P. Pharma, Inc.) and their service providers who worked very hard to execute and complete one of the largest CINV studies to date. Erin O’Boyle was an employee at A.P. Pharma at the initiation of the manuscript and is currently employed at FibroGen, Inc., 409 Illinois Street, San Francisco, CA 94158. Medical writing support was provided by Yvonne E. Yarker, PhD, CMPP, of SciStrategy Communications, and was funded by Heron Therapeutics, Inc. (formerly A.P. Pharma, Inc.).
Conflict of interest
Harry Raftopoulos has served in a consultant/advisory role for Merck & Co. Ralph Boccia has received clinic funding for the trial only. William Cooper has served in a consultant/advisory role for TFS International. Nashat Gabrail has no financial disclosures or potential conflicts of interest to report. Erin O’Boyle has served in a consultant/advisory role and has stock ownership as a previous employee of A.P. Pharma. Richard J. Gralla is a consultant and advisor to Merck & Co, to Helsinn, and to Eisai Inc. The authors declare that they have full control of the primary data and agree to allow the journal to review these data.
Funding
This study was sponsored by Heron Therapeutics, Inc (formerly A.P. Pharma, Inc.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Raftopoulos, H., Cooper, W., O’Boyle, E. et al. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Support Care Cancer 23, 723–732 (2015). https://doi.org/10.1007/s00520-014-2400-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2400-3