Abstract
Objectives
This is a retrospective observational study evaluating hypersensitivity reactions captured by an electronic medical record (EMR) system for weekly paclitaxel infusions.
Materials and methods
The study evaluates the demographics, co-morbidities, premedications, chemotherapy agents, and postmedications of patients reporting a hypersensitivity reaction to weekly infusions of paclitaxel at a major cancer center, from June 2006 to December 2007. Data were analyzed using descriptive statistics and logistic regression. P values <0.05 were considered significant.
Results
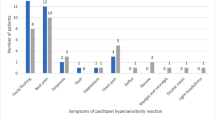
There were 51 hypersensitivity reactions in 36 patients during this time period that were documented in the allergy section of the EMR. Reactions occurred in patients with an average age of 55 years (SD = 10.77); 47 (92%) of the reactions occurred in females, and 42 (82%) of the reactions occurred with orders on the breast medical/surgical service. Thirty-two (63%) reactions occurred with either the first or second dose of weekly paclitaxel infusion. The most common premedication was dexamethasone (50 infusions), followed by diphenhydramine (18 infusions), and cimetidine (14 infusions). Thirty-three (65%) infusions had only one premedication. Postreaction, 41 (80%) cases had diphenhydramine and 30 (59%) cases had hydrocortisone administered prior to restarting the infusion. Logistic regression analysis did not indicate any relationship between history of previous allergies, hypertension, coronary disease, or chronic obstructive pulmonary disease and the number of premedications.
Conclusions
The results indicate that there is substantial variability in the type and number of premedications utilized in the management of paclitaxel hypersensitivity reactions. Interventions are needed to decrease the rate of hypersensitivity reactions from weekly paclitaxel infusions.

Similar content being viewed by others
References
Akerly W, Sikov WM, Cummings F et al (1997) Weekly high-dose paclitaxel in metastatic and locally advanced breast cancer. A preliminary report. Semin Oncol 24:87–90
Chang AY, Boros L, Asbury R et al (1997) Dose-escalation study of weekly 1-hour paclitaxel administration in patients with refractory cancers. Semin Oncol 24:69–71
Dyer AR, Stamler J, Berkson DM et al (1975) High blood-pressure: a risk factor for cancer mortality? Lancet 1:1051–1056. doi:10.1016/S0140-6736(75)91826-7
Goon PKY, Messerli FH, Lip GYH (2006) Hypertension and breast cancer: an association revisited? J Hum Hypertens 20:722–724. doi:10.1038/sj.jhh.1002053
Itoh Y, Sendo T, Hirakawa T et al (2004) Role of sensory nerve peptides rather than mast cell histamine in paclitaxel hypersensitivity. Am J Respir Crit Care Med 169:113–119. doi:10.1164/rccm.200307-901OC
Lau DHM, Ryu J, Gandara D et al (1999) Concurrent twice weekly paclitaxel and thoracic irradiation for stage III non-small cell lung cancer. Semin Radiat Oncol 9:117–120
Lenz HJ (2007) Management and preparedness for infusion and hypersensitivity reactions. Oncologist 12:601–609. doi:10.1634/theoncologist.12-5-601
Milani A, DePas T, Noberasco C et al (2007) A 15-min premedication for 1-h paclitaxel infusion: optimizing patients’ care. Lung Cancer 58:300–301. doi:10.1016/j.lungcan.2007.08.009
National Cancer Institute (2006) Common terminology criteria for adverse events v3.0 (CTCAE). National Cancer Institute, Bethesda
Quock J, Dea G, Tanaka M et al (2002) Premedication strategy for weekly paclitaxel. Cancer Invest 20:666–672. doi:10.1081/CNV-120003535
Rowinsky EK, Onetto N, Canetta RM et al (1992) Taxol: the first of the taxanes, an important new class of antitumor agents. Semin Oncol 19:646–662
Ruiz-Casado A, Calzas J, Garcia J et al (2006) Life-threatening adverse drug reaction to paclitaxel. Postmarketing surveillance. Clin Transl Oncol 8:60–61. doi:10.1007/s12094-006-0098-5
Sparano JA, Wang M, Martino D et al (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663–671
University of Texas M. D. Anderson (2008) Paclitaxel. Lexi-Comp ONLINE, Hudson
Weiss RB, Donehower RC, Wiernik PH et al (1990) Hypersensitivity reaction from taxol. J Clin Oncol 7:1263–1268
Zidan J, Hussein O, Abzah A et al (2008) Oral premedication for the prevention of hypersensitivity reactions to paclitaxel. Med Oncol 25:274–278
Acknowledgements
Presented as an invited lecture at the Supportive Care in Cancer MASCC/ISOO 2008 International Symposium in Houston, TX, USA on June 26–28, 2008. No funding was available for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lal, L.S., Gerber, D.L., Lau, J. et al. Retrospective evaluation of weekly paclitaxel hypersensitivity reactions reported utilizing an electronic medical record system at a tertiary cancer center. Support Care Cancer 17, 1311–1315 (2009). https://doi.org/10.1007/s00520-009-0588-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-009-0588-4