Abstract
Purpose
Recently, generic drugs of paclitaxel have been commonly used mainly by economic reasons; however, predictive factors for toxicities are not fully determined. Hypersensitivity reaction (HSR) is one of the most important adverse events in the paclitaxel-based therapy, and sometimes leads to lethal condition. The aim of the study was to identify predictors for HSR in patients treated with paclitaxel-based regimens.
Methods
All the patients treated with chemotherapy including paclitaxel at our hospital between 1998 and 2013 were retrospectively evaluated. Clinicopathological factors of the patients that developed HSR and those without HSR were compared, and predictive factors for HSR were identified.
Results
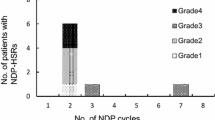
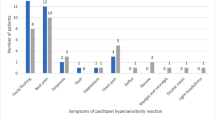
Among 414 patients enrolled in the study, 26 patients (6.3%) developed HSR. Multivariate analyses showed that younger age (odds ratio 6.31), a history of allergy (odds ratio 3.79), and short-course premedication (odds ratio 14.1) were identified as predictive factors for HSR. There was no significant difference in the incidence of HSR between original paclitaxel and generic drug. The incidence of HSR was higher as the number of these predictors was accumulated.
Conclusions
Three factors were identified as predictive factors for HSR: younger age, a history of allergy, and short-course premedication. Accumulation of these factors increased the incidence of HSR; however, the use of generic drug was not associated HSR in gynecologic cancer patients.


Similar content being viewed by others
References
Rowinsky EK, Donehower RC (1995) Paclitaxel (taxol). N Engl J Med 332:1004–1014
Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, Baker JR Jr, Van Echo DA, Von Hoff DD, Leyland-Jones B (1990) Hypersensitivity reactions from taxol. J Clin Oncol 8:1263–1268
Kloover JS, den Bakker MA, Gelderblom H, van Meerbeeck JP (2004) Fatal outcome of a hypersensitivity reaction to paclitaxel: a critical review of premedication regimens. Br J Cancer 90:304–305
Grosen E, Siitari E, Larrison E, Tiggelaar C, Roecker E (2000) Paclitaxel hypersensitivity reactions related to bee-sting allergy. Lancet 355:288–289
Sendo T, Sakai N, Itoh Y, Ikesue H, Kobayashi H, Hirakawa T, Nakano H, Oishi R (2005) Incidence and risk factors for paclitaxel hypersensitivity during ovarian cancer chemotherapy. Cancer Chemother Pharmacol 56(1):91–96
Piovano E, Pivetta E, Modaffari P, Martra F, Baima Poma C, Perotto S, Tripodi E, Zanfagnin V, Zola P, Ferrero A (2012) A search for predictive factors for hypersensitivity reactions to paclitaxel and platinum salts in chemotherapy for gynecologic pelvic neoplasms. Gynecol Obstet Invest 74:21–27
Markman M, Kennedy A, Webster K, Kulp B, Peterson G, Belinson J (2000) Paclitaxel-associated hypersensitivity reactions: experience of the gynecologic oncology program of the Cleveland Clinic Cancer Center. J Clin Oncol 18:102–105
Lasser EC, Walters A, Reuter SR, Lang J (1971) Histamine release by contrast media. Radiology 100:683–686
Lasser EC, Berry CC, Talner LB, Santini LC, Lang EK, Gerber FH, Stolberg HO (1987) Pretreatment with corticosteroids to alleviate reactions to intravenous contrast material. N Engl J Med 317:845–849
Bookman MA, Kloth DD, Kover PE, Smolinski S, Ozols RF (1997) Short-course intravenous prophylaxis for paclitaxel-related hypersensitivity reactions. Ann Oncol 8:611–614
Köppler H, Heymanns J, Weide R (2001) Dose reduction of steroid premedication for paclitaxel: no increase of hypersensitivity reactions. Onkologie 24:283–285
Chen W, Mempel M, Schober W, Behrendt H, Ring J (2008) Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy 63:1418–1427
Wolkewitz M, Rothenbacher D, Löw M, Stegmaier C, Ziegler H, Radulescu M, Brenner H, Diepgen TL (2007) Lifetime prevalence of self-reported atopic diseases in a population-based sample of elderly subjects: results of the ESTHER study. Br J Dermatol 156:693–697
Ratanajarusiri T, Sriuranpong V, Sitthideatphaiboon P, Poovoravan N, Vinayanuwat C, Parinyanitikul N, Angspatt P, Thawinwisan W, Tanasanvimon S (2016) A difference in the incidences of hypersensitivity reactions to original and generic taxanes. Chemotherapy 62(2):134–139
Markman M, Kennedy A, Webster K, Elson P, Peterson G, Kulp B, Belinson J (1999) Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol 17(4):1141
Acknowledgements
The authors thank MS. Hiromi Kubota for continuous contribution to our clinical study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study protocol was approved by the institutional review board of National Defense Medical College.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aoyama, T., Takano, M., Miyamoto, M. et al. Is there any predictor for hypersensitivity reactions in gynecologic cancer patients treated with paclitaxel-based therapy?. Cancer Chemother Pharmacol 80, 65–69 (2017). https://doi.org/10.1007/s00280-017-3332-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3332-7