Abstract
Background
Granisetron is a safe and effective prophylaxis for nausea and vomiting associated with moderate to highly emetogenic chemotherapy. Few trials have been conducted to determine the optimal effective dose of granisetron in children with cancer. The objective of this report was to compare two doses of granisetron in patients with optic pathway tumors receiving moderately emetogenic doses of carboplatin.
Patients and methods
In this double-blind, crossover, randomized study, antiemetic efficacy and tolerability of two dose levels (10 and 40 μg/kg) of granisetron in the prevention of acute and delayed nausea/emesis were compared in children and young adults. A total of 18 patients (13 boys) aged 1–23 years (median 7.7 years) treated with a moderately emetogenic dose of carboplatin were randomly assigned to receive either 10 or 40 μg/kg of slow granisetron intravenous (i.v.) infusions at alternating cycles of chemotherapy in a blinded fashion until the end of the study period or until their chemotherapy regimen ended. In this way, the patients acted as their own controls.
Results
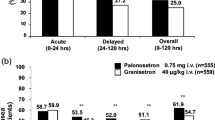
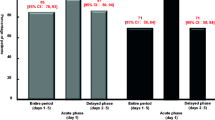
Patients in the granisetron 10 and 40 μg/kg groups received 104 and 121 cycles of chemotherapy, respectively. There was no significant difference in antiemetic efficacy in terms of nausea and emesis between the dose groups in the first 5 days of chemotherapy. The treatment was well tolerated.
Conclusion
We conclude that granisetron 10 and 40 μg/kg have comparable efficacy in controlling carboplatin-induced acute and delayed nausea/emesis and is well tolerated in children and young adults.
Similar content being viewed by others
References
ASHP (1999) Therapeutic guidelines on the pharmacologic management of nausea and vomiting in adult and pediatric patients receiving chemotherapy or radiation therapy or undergoing surgery. Am J Health-Syst Pharm 56:729–764
Aapro M (2004) Granisetron: an update on its clinical use in the management of nausea and vomiting. Oncologist 9:673–686
Aapro MS, Thuerlimann B, Sessa C et al; Swiss Research Group for Clinical Cancer Research (2003) A randomized double-blind trial to compare the clinical efficacy of granisetron with metoclopramide, both combined with dexamethasone in the prophylaxis of chemotherapy-induced delayed emesis. Ann Oncol 14:291–297
Aksoylar S, Akman SA, Ozgenc F et al (2001) Comparison of tropisetron and granisetron in the control of nausea and vomiting in children receiving combined cancer chemotherapy. Pediatr Hematol Oncol 18:397–406
Craft AW, Price L, Eden OB et al (1995) Granisetron as antiemetic therapy in children with cancer. Med Pediatr Oncol 25:28–32
de Boer-Dennert M, de Wit R, Schmitz PI et al (1997) Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer 76:1055–1061
Gandara D (1991) Progress in the control of acute and delayed emesis induced by cisplatin. Eur J Cancer 27:S9–S11 (discussion S22)
Gandara DR, Roila F, Warr D et al (1998) Consensus proposal for 5HT3 antagonists in the prevention of acute emesis related to highly emetogenic chemotherapy. Dose, schedule, and route of administration. Support Care Cancer 6:237–243
Gralla RJ, Osoba D, Kris MG et al (1999) Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical Oncology. J Clin Oncol 17:2971–2994
Gregory RE, Ettinger DS (1998) 5-HT3 receptor antagonists for the prevention of chemotherapy-induced nausea and vomiting. A comparison of their pharmacology and clinical efficacy. Drugs 55:173–189
Hahlen K, Quintana E, Pinkerton CR et al (1995) A randomized comparison of intravenously administered granisetron versus chlorpromazine plus dexamethasone in the prevention of ifosfamide-induced emesis in children. J Pediatr 126:309–313
Herrstedt J, Koeller JM, Roila F et al (2005) Acute emesis: moderately emetogenic chemotherapy. Support Care Cancer 13:97–103
Holdsworth MT, Raisch DW, Frost J (2006) Acute and delayed nausea and emesis control in pediatric oncology patients. Cancer 106:931–940
Jacobson SJ, Shore RW, Greenberg M et al (1994) The efficacy and safety of granisetron in pediatric cancer patients who had failed standard antiemetic therapy during anticancer chemotherapy. Am J Pediatr Hematol/Oncol 16:231–235
Kamanabrou D (1992) Intravenous granisetron-establishing the optimal dose. The Granisetron Study Group. Eur J Cancer 28A:S6–S11
Koizumi W, Tanabe S, Nagaba S et al (2003) A double-blind, crossover, randomized comparison of granisetron and ramosetron for the prevention of acute and delayed cisplatin-induced emesis in patients with gastrointestinal cancer: is patient preference a better primary endpoint? Chemotherapy 49:316–323
Komada Y, Matsuyama T, Takao A et al (1999) A randomised dose-comparison trial of granisetron in preventing emesis in children with leukaemia receiving emetogenic chemotherapy. Eur J Cancer 35:1095–1101
Kris MG, Hesketh PJ, Herrstedt J et al (2005) Consensus proposals for the prevention of acute and delayed vomiting and nausea following high-emetic-risk chemotherapy. Support Care Cancer 13:85–96
Lemerle J, Amaral D, Southall DP et al (1991) Efficacy and safety of granisetron in the prevention of chemotherapy-induced emesis in paediatric patients. Eur J Cancer 27:1081–1083
Luisi FA, Petrilli AS, Tanaka C et al (2006) Contribution to the treatment of nausea and emesis induced by chemotherapy in children and adolescents with osteosarcoma. Sao Paulo Med J 124:61–65
Mabro M, Cohn R, Zanesco L et al (2000) Oral granisetron solution as prophylaxis for chemotherapy-induced emesis in children: double-blind study of 2 doses. Bull Cancer 87:259–264 (French)
Matsui K, Fukuoka M, Takada M et al (1996) Randomised trial for the prevention of delayed emesis in patients receiving high-dose cisplatin. Br J Cancer 73:217–221
Miyajima Y, Numata S, Katayama I et al (1994) Prevention of chemotherapy-induced emesis with granisetron in children with malignant diseases. Am J Pediatr Hematol/Oncol 16:236–241
Navari R, Gandara D, Hesketh P et al (1995) Comparative clinical trial of granisetron and ondansetron in the prophylaxis of cisplatin-induced emesis. The Granisetron Study Group. J Clin Oncol 13:1242–1248
Orchard PJ, Rogosheske J, Burns L et al (1999) A prospective randomized trial of the antiemetic efficacy of ondansetron and granisetron during bone marrow transplantation. Biol Blood Marrow Transplant 5:386–393
Redmond K (1996) Advances in supportive care. Eur J Cancer Care (Engl) 4:1–7
Riviere A (1994) Dose finding study of granisetron in patients receiving high-dose cisplatin chemotherapy. The Granisetron Study Group. Br J Cancer 68:967–971
Soukop M (1994) A dose-finding study of granisetron, a novel antiemetic, in patients receiving high-dose cisplatin. Granisetron Study Group. Support Care Cancer 2:177–183
Tsuchida Y, Hayashi Y, Asami K et al (1999) Effects of granisetron in children undergoing high-dose chemotherapy: a multi-institutional, cross-over study. Int J Oncol 14:673–679
Yalcin S, Tekuzman G, Baltali E et al (1999) Serotonin receptor antagonists in prophylaxis of acute and delayed emesis induced by moderately emetogenic, single-day chemotherapy: a randomized study. Am J Clin Oncol 22:94–96
Acknowledgment
This work was presented in part at the 38th Annual Conference of the International Society of Paediatric Oncology (SIOP) 2006, Geneva, Switzerland, 18–21 September 2006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berrak, S.G., Ozdemir, N., Bakirci, N. et al. A double-blind, crossover, randomized dose-comparison trial of granisetron for the prevention of acute and delayed nausea and emesis in children receiving moderately emetogenic carboplatin-based chemotherapy. Support Care Cancer 15, 1163–1168 (2007). https://doi.org/10.1007/s00520-007-0242-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-007-0242-y