Abstract
Background
The authors conducted a retrospective study to determine whether dexrazoxane (ICRF-187) would reduce late anthracycline-induced cardiotoxicity in patients treated in childhood for hematological malignancy.
Patients and methods
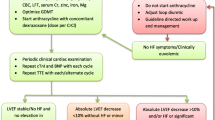
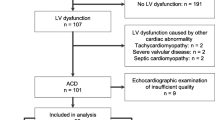
The authors examined 108 patients (63 male, 45 female) 5–29 years old, (median 15 years). All patients were in long-term remission of their malignancy. The cardioprotection was given to 68 patients (39 male, 29 female), and standard treatment was used in 40 patients (24 male, 16 female). Dexrazoxane (cardioxane, Chiron Company, The Netherlands) was given in 20:1 ratio to anthracycline. The follow-up time was 2–20 years (mean 7 years). The control group consisted of 41 volunteers (22 males, 19 females) 4–31 years old (median 18 years). The cardiotoxicity has been defined as the presence of heart failure or the decline of shortening fraction below 30% or ejection fraction (EF) below 55%. The end-systolic wall stress (ESS), myocardial performance index (MPI; Tei index), and parameters of left ventricular diastolic filling were also assessed.
Results
The anthracycline cardiomyopathy with the presence of heart failure was diagnosed in only one patient treated with a standard regimen. The pathological decline of fractional shortening was present in three (5%) and six (15%) patients with and without cardioprotection given, respectively. Similarly, none of the patients with cardioprotection revealed a pathological value of EF, while four (10%) patients without cardioprotection showed an EF decrease. Finally, ESS and isovolumic relaxation time were pathologically increased in the group without cardioprotection in comparison to the controls and to the group with cardioprotection. However, the MPI was significantly increased in both groups of patients.
Conclusions
Dexrazoxane reduces the risk of late clinical and subclinical cardiotoxicity and does not affect the response rates to chemotherapy and overall survival during the median follow-up period of 7 years (follow-up period 2–20 years).


Similar content being viewed by others
References
Allen TM (1998) Oncologic agents in sterically stabilized liposomes: basic considerations. Long circulating liposomes: old drugs, new therapeutics. Springer, Berlin Heidelberg New York, pp 19–28
Basser RL, Green MD (1993) Strategies for prevention of anthracycline cardiotoxicity. Cancer Treat Rev 19:57–77
Bristow MR, Billingham ME, Mason JW, Daniels JR (1978) Clinical spectrum of anthracycline antibiotic cardiotoxicity. Cancer Treat Rep 62:873–879
Bu’Lock FA, Gabriel HM, Oakhill A, Mott MG, Martin RP (1993) Cardioprotection by ICRF-187 against high dose anthracycline toxicity in children with malignant disease. Br Heart J 70:185–188
Bu’Lock FA, Mott MG, Oakhill A, Martin P (1995) Left ventricular diastolic function after anthracycline chemotherapy in childhood: relation with systolic function, symptoms, and pathophysiology. Br Heart J 73:340–350
Bu’Lock FA, Mott MG, Oakhill A, Martin RP (1999) Left ventricular diastolic filling patterns associated with progressive anthracycline-induced myocardial damage: a prospective study. Pediatr Cardiol 20:252–263
Eidem BW, Sapp BG, Suarez CR, Cetta F (2001) Usefulness of the myocardial performance index for early detection of anthracycline-induced cardiotoxicity in children. Am J Cardiol 87:1120–1122
Ewer MS, Jaffe N, Ried H, Zietz HA, Benjamin RS (1998) Doxorubicin cardiotoxicity in children: comparison of a consecutive divided daily dose administration schedule with single dose (rapid) infusion administration. Med Pediatr Oncol 31:512–515
Gharib MI, Burnett AK (2002) Chemotherapy-induced cardiotoxicity: current practice and prospects of prophylaxis. Eur J Heart Fail 4:23–242
Giantris A, Abdurrahman L, Hinkle A, Asselin B, Lipshultz SE (1998) Anthracycline-induced cardiotoxicity in children and young adults. Crit Rev Oncol/Hematol 4:53–68
Hasinoff BB, Hellmann K, Herman EH, Ferrans VJ (1998) Chemical, biological and clinical aspects of dexrazoxane and other bisdioxopiperazines. Curr Med Chem 5:1–28
Iarussi D, Indolfi P, Casale F, Coppolino P, Tedesco MA, DiTullio MT (2001) Recent advances in the prevention of anthracycline cardiotoxicity in childhood. Curr Med Chem 8:1649–1660
Iarussi D, Galderisi M, Raitti G, Tedesco MA, Indolfi P, Casale F, DiTullio MT, DeDinitiis O, Iacono A (2001) Left ventricular systolic and diastolic function after anthracycline chemotherapy in childhood. Clin Cardiol 24:663–669
Johnson GL, Moffett CB, Geil JD, Greenwood MF, Noonam JA (1996) Late echocardiographic findings following childhood chemotherapy with normal serial cardiac monitoring. J Pediatr Hematol/Oncol 18:72–75
Koning J, Palmer P, Franks CR, Mulder DE, Speyer JL, Green MD, Hellmann K (1991) Cardioxane-ICRF-187. Towards anticancer drug specificity through selective toxicity reduction. Cancer Treat Rev 18:1–19
Lippens RJJ (1999) Liposomal daunorubicin (DaunoXome) in children with recurrent or progressive brain tumors. Pediatr Hematol Oncol 16:131–139
Lipshultz SE, Colan SD, Gelber RD, Peres-Atayde AR, Sallan SE, Sanders SP (1991) Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med 324:808–815
Lipschultz SE, Lipsitz SR, Mone SM et al (1995) Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med 332:1738–1743
Lipshultz SE, Giantris AL, Lipsitz SR, Dalton VC, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Mogharbi A, Samson Y, Schorin SM, Gelber RD, Sallan SE, Colan SD (2002) Doxorubicin administration by continuous infusion is not cardioprotective: the dana-farber 91-01 acute lymphoblastic leukemia protocol. J Clin Oncol 20:1677–1682
Lipshultz SE, Colan SD, Silverman LB, Levy DE, Dalton VK, Rifai N, Lipsitz SR, Gelber RD, Salan SE (2002) Dexrazoxane reduces incidence of doxorubicin-associated acute myocardiocyte injury in children with acute lymphoblastic leukemia (ALL). J Clin Oncol 15:1544–1552
Nysom K, Colan CD, Lipshultz SE (1998) Late cariotoxicity following anthracycline therapy for childhood cancer. Prog Pediatr Cardiol 8:121–138
Otto CM, Pearlman AS (1995) Textbook of clinical echocardiography, 1st edn. W.B. Saunders, Philadelphia, PA, USA
Rubio ME, Wiegman A, Naeff MS (1995) ICRF-187 (Cardioxane) protection against doxorubicin induced cardiomyopathy in pediatric osteosarcoma patients. Proc Am Soc Clin Oncol 14:1403a
Seymour L, Bramwell V, Moran LA (1999) Use of dexrazoxane as a cardioprotectant in patients receiving doxorubicin or epirubicin chemotherapy for the treatment of cancer. Cancer Prev Control 3:145–159
Shan K, Lincoff M, Young JB (1996) Anthracycline-induced cardiotoxicity. Ann Intern Med 125:47–58
Sharpe M, Easthope SE, Keating GM, Lamb HM (2002) Polyethylene glycol-liposomal doxorubicin. A review of its use in the management of solid and haematological malignancies and AIDS-related Kaposi’s sarcoma. Drugs 62:2089–2126
Schiavetti A, Castello MA, Versacci P, Varrasso G, Padula A, Ventriglia F, Werner B, Colloridi V (1997) Use of ICRF-187 for prevention of anthracycline cardiotoxicity in children: preliminary results. Pediatr Hematol Oncol 14:213–222
Schuchter LM, Hensley ML, Meropol NJ, Winer EP (2002) 2002 update of recommendations for the use of chemotherapy and radiotherapy protectants: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 20:2895–2903
Speyer J, Wasserheit C (1998) Strategies for reduction of anthracycline cardiac toxicity. Semin Oncol 25:525–537
Steinherz LJ, Steinherz PG, Tan CHTC, Heller G, Murphy ML (1991) Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA 266:1672–1677
Steinherz LJ, Graham T, Hurwitz R et al (1992) Guidelines for cardiac monitoring of children during and after anthracycline therapy: report of the Cardiology Committee of the Childrens Cancer Study Group. Pediatrics 89:942–948
Steinherz LJ, Steinherz PG, Tan CH (1995) Cardiac failure and dysrhythmias 6–19 years after anthracycline therapy: a series of 15 patients. Med Pediatr Oncol 24:352–361
Steinherz LJ, Wexler LH (1998) The prevention of anthracycline cardiomyopathy. Prog Pediatr Cardiol 8:97–107
Tei CH, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, Tajik AJ, Seward JB (1995) New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function—a study in normals and dilated cardiomyopathy. J Cardiol 26:357–366
Von Hoff DD, Rozencweig M, Layard M, Slavik M, Muggia FM (1977) Daunomycin-induced cardiotoxicity in children and adults. Am J Med 62:200–208
Von Hoff DD, Layard MW, Basa P, Davis HL, Von Hoff AL, Rozencweig M, Muggia FM (1979) Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 91:710–717
Wexler LH, Andrich MP, Venzon D, Berg SL, McClure LW, Chen CC, Dilsizian V, Avila N, Jarosinski P, Balis FM, Poplack DG, Horowitz ME (1996) Randomized trial of the cardioprotective agent ICRF-187 in pediatric sarcoma patients treated with doxorubicin. J Clin Oncol 14:362–372
Wiseman LR, Spencer CM (1998) Dexrazoxane. Drugs 65:385–403
Acknowledgements
This study was supported by grant IGA from the Ministry of Health No. NR/8006-3 and partially by a grant from the Ministry of Education, Youth and Sports of the Czech Republic No. 002 162 2402.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elbl, L., Hrstkova, H., Tomaskova, I. et al. Late anthracycline cardiotoxicity protection by dexrazoxane (ICRF-187) in pediatric patients: echocardiographic follow-up. Support Care Cancer 14, 128–136 (2006). https://doi.org/10.1007/s00520-005-0858-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-005-0858-8