Abstract
Background
Cardiotoxicity associated with anthracycline-based chemotherapies has limited their use in patients with preexisting cardiomyopathy or heart failure. Dexrazoxane protects against the cardiotoxic effects of anthracyclines, but in the USA and some European countries, its use had been restricted to adults with advanced breast cancer receiving a cumulative doxorubicin (an anthracycline) dose > 300 mg/m2. We evaluated the off-label use of dexrazoxane as a cardioprotectant in adult patients with preexisting cardiomyopathy, undergoing anthracycline chemotherapy.
Methods
Between July 2015 and June 2017, five consecutive patients, with preexisting, asymptomatic, systolic left ventricular (LV) dysfunction who required anthracycline-based chemotherapy, were concomitantly treated with off-label dexrazoxane, administered 30 min before each anthracycline dose, regardless of cancer type or stage. Demographic, cardiovascular, and cancer-related outcomes were compared to those of three consecutive patients with asymptomatic cardiomyopathy treated earlier at the same hospital without dexrazoxane.
Results
Mean age of the five dexrazoxane-treated patients and three patients treated without dexrazoxane was 70.6 and 72.6 years, respectively. All five dexrazoxane-treated patients successfully completed their planned chemotherapy (doxorubicin, 280 to 300 mg/m2). With dexrazoxane therapy, changes in LV systolic function were minimal with mean left ventricular ejection fraction (LVEF) decreasing from 39% at baseline to 34% after chemotherapy. None of the dexrazoxane-treated patients experienced symptomatic heart failure or elevated biomarkers (cardiac troponin I or brain natriuretic peptide). Of the three patients treated without dexrazoxane, two received doxorubicin (mean dose, 210 mg/m2), and one received daunorubicin (540 mg/m2). Anthracycline therapy resulted in a marked reduction in LVEF from 42.5% at baseline to 18%. All three developed symptomatic heart failure requiring hospitalization and intravenous diuretic therapy. Two of them died from cardiogenic shock and multi-organ failure.
Conclusion
The concomitant administration of dexrazoxane in patients with preexisting cardiomyopathy permitted successful delivery of anthracycline-based chemotherapy without cardiac decompensation. Larger prospective trials are warranted to examine the use of dexrazoxane as a cardioprotectant in patients with preexisting cardiomyopathy who require anthracyclines.
Similar content being viewed by others
Introduction
Since their introduction in the early 1950s, anthracyclines have been widely used for treating a variety of solid and hematologic cancers in both adults and children [1]. However, cardiotoxicity has limited their use in patients with preexisting cardiomyopathy or symptomatic heart failure [2]. In the early 1970s, Eugene Herman discovered that dexrazoxane reduced anthracycline-induced cardiotoxicity [3]. In 1995, the US Food and Drug Administration (FDA) approved dexrazoxane for cardioprotection in women receiving fluorouracil, doxorubicin, and cyclophosphamide (FDC) chemotherapy for breast cancer and who required a cumulative doxorubicin dose > 350 mg/m2 [4]. Similarly, in 2011 the European Medicines Agency (EMA) decided that the use of dexrazoxane should be restricted to adult breast cancer patients requiring a cumulative dose of doxorubicin > 300 mg/m2 or a cumulative dose of epirubicin > 540 mg/m2 [5]. This limitation comes from debatable concerns that dexrazoxane might reduce anti-tumor response rates and increase the risk of secondary hematologic malignancies [6,7,8,9].
We sought to determine whether dexrazoxane would be cardioprotective in patients without the current indications for its use. Here, we present a consecutive series of patients from a single institution with preexisting cardiomyopathy, who were treated with anthracyclines, and compare their cardiovascular and cancer-related outcomes to those of similar patients who subsequently received concomitant off-label dexrazoxane with anthracycline-based chemotherapy.
Methods
In the two years preceding the initiation of the Lahey cardio-oncology program (July 2013 to June 2015), we noted that several patients with either preexisting cardiomyopathy, or those who experienced anthracycline-induced cardiomyopathy, developed worsening LV systolic function and major adverse cardiovascular events including heart failure, cardiogenic shock, arrhythmias, and cardiovascular death, despite the use of maximally tolerated, guideline-directed heart failure therapy.
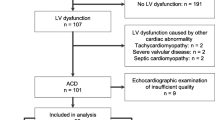
After evaluating the evidence on the use of dexrazoxane for cardioprotection against anthracycline-induced cardiotoxicity, we developed a clinical practice algorithm (Fig. 1) based on consensus among internal and external experts in cardio-oncology, hematology-oncology, and heart failure to use off-label concomitant dexrazoxane, in addition to guideline-directed heart failure therapy and close cardiac monitoring, in subsequent patients with preexisting cardiomyopathy who required anthracycline-based chemotherapy. Since this was a consensus-based clinical practice guideline, and not an experimental research study, institutional review board approval was not required.
Patient selection
Beginning in July 2015, five consecutive patients with preexisting asymptomatic LV systolic dysfunction, who required anthracycline-based chemotherapy, were treated with dexrazoxane as a cardioprotectant as per our consensus protocol. Three consecutive patients with preexisting asymptomatic LV systolic dysfunction who received anthracycline based chemotherapy between July 2013 and June 2015 were identified for comparison.
Cardioprotection protocol
All five patients received off-label dexrazoxane in a 10:1 ratio of dexrazoxane to doxorubicin, or at a reduced dose based on their creatinine clearance, 30 min before each anthracycline dose [4] throughout the therapy, regardless of cancer type or stage. All patients also received maximally tolerated, guideline-directed heart failure therapy [10]. Complete blood count, liver function, kidney function, and zinc, iron, and magnesium concentrations were assessed at baseline and after each chemotherapy cycle. Serum cardiac biomarkers including cardiac troponin I (cTnI) and brain natriuretic peptide (BNP) were measured before and within 48 h after each dose of anthracyclines [11,12,13], as well as 6 months after completion of chemotherapy. Echocardiograms were obtained at baseline, before every subsequent or alternate cycle of chemotherapy (as per the primary cardiology or cardio-oncology provider), immediately after the completion of anthracycline-based chemotherapy, and 6 months later [12].
Patient follow-up
All patients receiving dexrazoxane were followed in our cardio-oncology clinic, either before each subsequent or alternate cycle of anthracycline chemotherapy, as deemed appropriate by their providers. All patients were thoroughly examined for signs or symptoms of heart failure. We also collected data on cardiovascular and cancer-related outcomes (asymptomatic LV systolic dysfunction, heart failure, arrhythmias, cardiogenic shock, elevated serum cardiac biomarker concentrations defined as cTnI > 0.03 ng/mL and/or BNP > 250 pg/mL, tumor response, secondary malignancy, or death from any cause) during follow-up. Patients were followed until death or loss to follow-up.
Results
Patients treated without Dexrazoxane
Between July 2013 and June 2015, we had treated three consecutive male patients treated with anthracycline but without dexrazoxane (patients #1, #2, and #3; mean age, 72.6 years) at the Lahey Hospital and Medical Center. Patient #1 had preexisting asymptomatic non-ischemic cardiomyopathy (LVEF 40%), patient #2 had preexisting asymptomatic ischemic cardiomyopathy (LVEF 45%), and patient #3 had anthracycline-induced cardiomyopathy that developed after receiving a cumulative daunorubicin dose of 270 mg/m2 for acute myeloid leukemia (LVEF 45%) (Table 1). Each patient in this group had received maximally tolerated doses of guideline-directed heart failure therapy. Patients #1 and #2 received doxorubicin (cumulative doses of 300 mg/m2 and 120 mg/m2, respectively), and patient #3 received daunorubicin (cumulative dose 540 mg/m2).
All three patients in this group experienced marked reductions in LV systolic function (the mean LVEF decreased from 42.5% at baseline to 18% after chemotherapy), and developed symptomatic heart failure requiring hospitalization and intravenous diuretic therapy, either during or within the first six months after completion of chemotherapy (Table 2). Patients #1 and #2 developed fatal cardiogenic shock and multi-organ failure. Patient #2 had advanced, diffuse, large B-cell lymphoma and received only two cycles of anthracycline-based chemotherapy before experiencing heart failure, cardiogenic shock, and death. Patient #3 had acute myeloid leukemia and cardiomyopathy that developed during chemotherapy with daunorubicin (LVEF 45%). He continued therapy and received an additional 270 mg/m2 of daunorubicin with further worsening of his cardiomyopathy (LVEF 30%) that persisted, despite treatment with neurohormonal antagonists.
Patients treated with Dexrazoxane
The dexrazoxane protocol was implemented in the next five consecutive eligible patients (patients #4 through #8; mean age, 70.6 years), one man and four women, four with non-ischemic cardiomyopathy and one with ischemic cardiomyopathy. The mean pre-chemotherapy LVEF for these five dexrazoxane-treated patients was 39% (range, 35 to 45%) (Table 1). Patient #4 had an implantable cardioverter-defibrillator for primary prevention. All five patients received doxorubicin (280 to 300 mg/m2).
The median follow-up period was 13.5 months (range, 12–30 months). Two patients treated without dexrazoxane died and none were lost to follow-up.
The mean post-chemotherapy LVEF in this group was 34% and all five completed planned chemotherapy without any major adverse cardiovascular events (Table 2). Patient #5, with a preexisting left bundle branch block and non-ischemic cardiomyopathy (baseline LVEF 35%), required cardiac resynchronization therapy with a bi-ventricular implantable cardioverter-defibrillator after chemotherapy, given that her LVEF persistently remained < 35%. None of the patients experienced symptomatic heart failure requiring intravenous diuresis or hospitalization, and no secondary malignancies were detected ≥12 months after completion of chemotherapy.
Serum concentrations of cTnI and BNP were not elevated in any of the five patients. Patients #4 and #8 had sporadic episodes of non-sustained ventricular tachycardia, but no sustained arrhythmias were noted (Table 2).
Three of the five patients in this group experienced significant neutropenia (absolute neutrophil count < 500/μL), and two patients experienced neutropenic fever requiring treatment with antibiotics. None of the patients developed any detectable end-organ damage as a result. No clinically important abnormalities were noted in any other laboratory parameters including serum liver function, kidney function, and zinc, iron, and magnesium concentrations.
Discussion
The patients in our case series had preexisting cardiomyopathy that placed them at increased risk for cardiotoxicity from anthracycline-based chemotherapy. Off-label use of concomitant dexrazoxane as a cardioprotectant allowed successful administration of planned anthracycline-based chemotherapy, without symptomatic cardiac decompensation. In contrast, similar patients who received anthracyclines without cardioprotection, experienced a significant decline in their LVEF, coupled with adverse cardiovascular outcomes including death. It is also important to note that in our case series, dexrazoxane was not associated with either a reduced anti-tumor response or the development of secondary malignancies during the median follow-up period of 13.5 months. Our data, although limited, support the use of concomitant dexrazoxane as a cardioprotectant in patients with preexisting cardiomyopathy who require anthracycline therapy.
The overlap of Cancer and cardiomyopathy
With an aging population, and with an increase in the number of patients with cardiovascular disease (CVD) and cancer survivors, greater overlap between CVD and cancer is expected [14,15,16,17]. The risk of anthracycline-induced cardiotoxicity is increased in the elderly, patients with cardiovascular comorbidities, and in patients receiving additional cardiotoxic chemotherapies or thoracic irradiation [18]. Our case series includes patients with preexisting LV systolic dysfunction who are often excluded from receiving anthracycline-based chemotherapy due to the risk of worsening cardiomyopathy. This often results in inferior cancer outcomes, or in cases where they receive anthracyclines, increased cardiovascular morbidity and mortality. In this case series, we report our observations on the successful use of dexrazoxane as a cardioprotectant in this patient population.
Anthracycline-related cardiotoxicity
Anthracyclines have been widely used to treat a variety of solid and hematological cancers in children and adults since the 1950s [1]. Cardiac complications were reported a few years later [19], but anthracyclines remain one of the most commonly used classes of chemotherapeutic agents, given their oncologic efficacy [1].
Anthracycline-related cardiotoxicity ranges from subclinical cardiomyopathy to florid heart failure. Cardiotoxicity may occur within the first week of anthracycline treatment or even decades later [20]; however, most cases occur within the first year after treatment [21]. The frequency and characteristics of both clinical and subclinical cardiotoxicity may vary between children and adults [22]. In adult breast cancer patients, the incidence of cardiotoxicity is approximately 9% [21]. The actual incidence of late cardiotoxicity in long-term survivors of childhood cancer is difficult to ascertain since it increases with longer survival, but up to one third of childhood survivors have some evidence of cardiac dysfunction, including overt heart failure [23].
Doxorubicin is not the only anthracycline associated with cardiotoxicity; epirubicin and daunorubicin, other commonly used anthracyclines to treat several cancers, have also caused similar cardiotoxicity, especially at high doses [24,25,26]. In this case series as well, one of the patients in the anthracycline-treated group who did not receive dexrazoxane (patient #5) experienced daunorubicin-related cardiotoxicity resulting in cardiomyopathy and heart failure.
Anthracyclines are believed to cause cardiotoxicity by multiple mechanisms; including both the generation of oxygen free radicals and by inhibition of topoisomerase 2B. Substantial experimental data support the involvement of iron in anthracycline-induced cardiotoxicity, including evidence suggesting the importance of the HFE gene in regulating cardiac iron accumulation after exposure to anthracyclines, irrespective of a patient’s systemic iron load [27].
Preventing cardiomyopathy
Dexrazoxane: Mechanism of action
Dexrazoxane is an iron chelator that binds free iron or removes iron from the doxorubicin-iron complex, thereby preventing oxygen free radical formation. This property may explain how dexrazoxane reduces some of the cardiotoxic effects of anthracyclines [28]. However, other iron chelators, such as deferasirox, or ICRF-161, are not cardioprotective [29, 30]. Dexrazoxane also changes the configuration of topoisomerase II [31] thus preventing anthracyclines from binding to the topoisomerase II complex [32]. This may be an additional mechanism for the cardioprotective effects of dexrazoxane.
Dexrazoxane: Facts and fiction (Table 3)
The current approved use of dexrazoxane in adults is based on two multicenter, randomized controlled trials (088001 and 088006) involving more than 500 women with advanced breast cancer [6]. In these studies, women were randomized to receive doxorubicin, with or without dexrazoxane (10:1 dexrazoxane:doxorubicin). In study 088001, 15% of women who received dexrazoxane and 31% of those who received placebo experienced a cardiac event (HR 2.63, 95%CI 1.61 to 4.27). Similarly, in study 088006, 14% of those treated with dexrazoxane experienced a cardiovascular event compared to 31% treated with placebo (HR 2.0, 95% CI 1.01 to 3.96). Cardiac events were defined as a decline in LVEF from baseline ≥10% and below the institution’s lower limit of normal (LLN), a decline in LVEF of ≥20% from baseline, or the development of heart failure (with two or more of the following: cardiomegaly established by radiography, basilar rales, S3 gallop, or paroxysmal nocturnal dyspnea, orthopnea, or significant dyspnea on exertion) [6]. The early signal of substantial cardioprotection in these studies, led to the use of open-label dexrazoxane in patients on placebo who had received a cumulative doxorubicin dose of 300 mg/m2. These patients experienced a marked reduction in cardiovascular events, despite the late introduction of dexrazoxane (HR 3.5, 95% CI 2.2 to 5.7) [6, 7].
In trial 088001, the dexrazoxane group had a lower antitumor response rate (complete remission and partial remission) when compared to the placebo group (48% vs. 63%, P = 0.007), although the overall time-to-progression and overall survival were not affected. Response rates were not significantly different in trial 088006. However, despite these disparate results, this led to a concern that dexrazoxane may reduce the anti-tumor efficacy of anthracyclines. Furthermore, in studies of children receiving dexrazoxane, there was an initial concern of an increased incidence of secondary malignancies, such as acute myeloid leukemia and myelodysplastic syndrome. Further follow-up of those same study patients has subsequently found that the concern for reduced oncologic efficacy was not correct [8]. Because the maximum benefit of dexrazoxane was seen in patients receiving a cumulative doxorubicin dose ≥300 mg/m2, and out of concern for reduced anti-tumor efficacy as well as increased risk of secondary malignancy, the FDA and EMA restricted its use to adults with advanced breast cancer receiving higher cumulative doses of doxorubicin [4,5,6,7,8]. The most recent American Society of Clinical Oncology guidelines also recommend limiting the use of dexrazoxane to patients receiving a cumulative doxorubicin dose > 250 mg/m2 [33].
Although dexrazoxane is not approved as a cardioprotectant in adults with hematologic malignancies, it has nevertheless been used in these patients. A retrospective analysis of adults with non-Hodgkin lymphoma treated with anthracycline-based combination chemotherapy revealed that dexrazoxane had similar cardioprotective effects [34, 35]. Dexrazoxane has also been shown to be cardioprotective in elderly patients with acute myeloid leukemia or small cell lung cancer [36, 37]. Similarly, dexrazoxane provided marked cardioprotection against epirubicin-related cardiotoxicity in patients with breast cancer or sarcoma with no evidence of reduced antitumor activity [38, 39].
Dexrazoxane has also been successfully used in children with various solid and hematologic cancers. The Dana-Farber Cancer Institute’s childhood acute lymphoblastic leukemia consortium protocols from 1995 to 2001 found that dexrazoxane reduced or prevented doxorubicin-induced cardiac injury in children with high-risk acute lymphoblastic leukemia, as indicated by lower serum cardiac troponin T concentrations and preserved LV systolic function and structure without reducing oncologic efficacy [3]. This has been found in multiple additional pediatric studies, including those from the Children’s Oncology Group [40, 41].
A single study is responsible for the unfounded fear that dexrazoxane reduces the oncologic efficacy of anthracyclines in adults, especially when used from the beginning of anthracycline therapy [6]. The results of this study have not been confirmed in other randomized trials and retrospective studies in children and adults with various cancers [40, 41].
As previously mentioned, secondary malignancies, such as acute myeloid leukemia and myelodysplastic syndrome, have been reported in studies of children receiving dexrazoxane [8]. However, this increased risk of secondary malignancies was only observed in two randomized controlled trials that administered etoposide with doxorubicin and dexrazoxane; in other randomized trials that did not include etoposide, the risk of acute myeloid leukemia or other secondary malignancies was not higher in the dexrazoxane arm [9]. Longer follow-up of the same study patients with the initial concern about secondary malignancies did not confirm this initial concern [40]. Similarly, a single randomized trial that administered cranial radiation to all patients (with either T-cell acute lymphoblastic leukemia or non-Hodgkin lymphoma) reported an increased rate of secondary brain tumors in the dexrazoxane arm. No brain tumors developed in any of the 717 dexrazoxane-treated patients enrolled in the other studies [9]. Overall, the concern for a higher incidence of secondary malignancies with dexrazoxane use is unwarranted. In retrospective analyses of long-term survivors of both childhood and adult cancers, dexrazoxane was not associated with an increase in secondary malignancies when used with doxorubicin chemotherapy [9, 41, 42]. Long-term follow-up (median follow-up, 12.6 years) of Children’s Oncology Group trials has established that dexrazoxane neither reduces long-term overall survival nor increases deaths from the original cancer [40]. In fact, dexrazoxane use in pediatric acute myeloid leukemia (AML) patients has shown better 3-year overall survival and event free survival [43].
Based on the efficacy of dexrazoxane as a cardioprotective agent in patients with a wide variety of cancers and with evolving safety data demonstrating that it does not impair chemotherapeutic efficacy and is not associated with an increased risk of secondary malignancies, in 2015 the European marketing authorization holder for Cardioxane (dexrazoxane; Clinigen Healthcare Ltd., Staffordshire, UK) submitted an application to all relevant national regulatory authorities to widen the indication from “advanced and/or metastatic adult breast cancer patients” to “cancer patients” and remove the contraindication in children and adolescents. The proposal to widen the indication was declined. However, after a careful review of dexrazoxane’s risk-benefit profile, the contraindication was lifted for children and adolescents requiring high doses (≥300 mg/m2) of anthracyclines [44].
Baseline LVEF is a significant predictor of anthracycline-induced cardiotoxicity [21]. Furthermore, while anthracycline-induced cardiotoxicity is dose-dependent, there is no threshold dose below which cardiotoxicity is not observed [11]. Thus, patients with preexisting cardiomyopathy are more susceptible to developing cardiotoxicity, even at lower anthracycline doses, and are often excluded from receiving anthracyclines altogether [18, 45]. Dexrazoxane could be particularly useful in these patients, but its use is limited by the current FDA and EMA label [4]. In this case series, we demonstrate that the off-label use of concomitant dexrazoxane in patients with preexisting, asymptomatic LV dysfunction, allows successful administration of anthracycline-based chemotherapy without adverse cardiovascular outcomes.
Future perspective
Immunotherapies, including checkpoint inhibitors and adoptive cellular therapy, as well as targeted therapy, are now approved for several cancers and will likely change the landscape of oncology in coming years [46,47,48]. However, many patients will still need anthracycline chemotherapy to be cured. Cardiotoxicity is a well-known side effect of anthracyclines. Patients with preexisting cardiomyopathy are at the highest risk for anthracycline-related cardiotoxicity and are often denied lifesaving or sustaining anthracycline-based chemotherapy, even when there is no other alternative [18, 45]. Cardioprotection by dexrazoxane may offer a modality by which high-risk patients can receive anthracyclines without significantly increased cardiovascular morbidity and mortality [44].
Given that anthracycline-related cardiotoxicity can occur at low doses in patients with preexisting cardiomyopathy, our case series supports the use of dexrazoxane in this high-risk population from the beginning of anthracycline therapy, rather than after a cumulative dose of 300 mg/m2 is reached. Furthermore, we would advocate expanding its use to all cancers and not limiting it to patients with advanced breast cancer receiving doxorubicin. In patients with normal LV systolic function, the decision to administer dexrazoxane from the beginning of anthracycline therapy versus after receiving a cumulative dose of 300 mg/m2 may be debatable, but using dexrazoxane from the initiation of anthracycline therapy should be considered, regardless of the type of cancer [49].
Although dexrazoxane is cardioprotective, its protection is not complete and these patients should be monitored closely for signs of clinical and even subclinical cardiotoxicity. Although there is no question that signs of clinically significant cardiotoxicity may lead to interruption or reduction of anthracycline therapy, there is no consensus among our authors, or many in this field, that withholding or altering anthracycline therapy based solely on changes in subclinical cardiovascular status (changes in LVEF or serum cardiac biomarkers) will lead to a higher quality of life for these patients.
Strengths and limitations of the study
This is a pragmatic study under real-world conditions. Although bias is possible in any case series, by including consecutive patients who met inclusion and exclusion criteria and comparing the outcomes with a historical group of anthracycline-treated patients who did not receive dexrazoxane, we attempted to minimize bias. Our objectives were clear, the outcomes were clinically relevant, and the data were prospectively collected. Finally, there was no loss to follow-up in either group. However, we recognize the limitations of findings based on only a few patients from a single center. Data collection in the patients treated earlier without dexrazoxane was limited by its retrospective nature.
Conclusion
Our results, albeit limited, support the use of concomitant dexrazoxane for cardioprotection in patients with preexisting cardiomyopathy from the beginning of anthracycline therapy, regardless of cancer type or stage. Larger prospective trials are warranted to examine the expanded use of dexrazoxane in high-risk patients, including those with preexisting cardiomyopathy; cancers other than those affecting the breast; and without a minimum cumulative anthracycline dose.
Abbreviations
- BNP:
-
Brain natriuretic peptide
- cTnI:
-
Cardiac troponin I
- CVD:
-
Cardiovascular disease
- EMA:
-
European Medical Agency
- FDA:
-
Food and Drug Administration
- LV:
-
Left ventricular
- LVEF:
-
Left ventricular ejection fraction
References
Volkova M, Russell R 3rd. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev. 2011;7(4):214–20.
Doxorubicin Hydrochloride for Injection, USP [package insert]. Pharmacia & Upjohn Company, Division of Pfizer Inc., NY, NY 10017. 2010.
Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351(2):145–53.
Dexrazoxane (Zinecard) [package insert]. Pfizer Injectables. Division of Pfizer Inc., NY, NY 10017. 2014.
2011 EMAArD-cmpEHA-. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Dexrazoxane_31/WC500120340.pdf. Accessed 23 July 2018.
Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol. 1997;15(4):1318–32.
Swain SM, Whaley FS, Gerber MC, Ewer MS, Bianchine JR, Gams RA. Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin-containing therapy. J Clin Oncol. 1997;15(4):1333–40.
Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol. 2007;25(5):493–500.
Shaikh F, Dupuis LL, Alexander S, Gupta A, Mertens L, Nathan PC. Cardioprotection and second malignant neoplasms associated with dexrazoxane in children receiving anthracycline chemotherapy: a systematic review and meta-analysis. J Natl Cancer Inst. 2016;108(4).
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137–e61.
Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109(22):2749–54.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–39.
Okumura H, Iuchi K, Yoshida T, Nakamura S, Takeshima M, Takamatsu H, et al. Brain natriuretic peptide is a predictor of anthracycline-induced cardiotoxicity. Acta Haematol. 2000;104(4):158–63.
Ganatra S, Hayek SS. Cardio-oncology for GenNext: a missing piece of the training puzzle. J Am Coll Cardiol. 2018;71(25):2977–81.
Vincent GK, Velkoff VA, US Census Bureau. The next four decades. The older population in the United States: 2010 To 2050. Cur Pop Reports. Available at: https://www.census.gov/prod/2010pubs/p25-1138.pdf. Accessed 30 April 2018
DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–71.
Writing Group M, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–360.
Groarke JD, Nohria A. Anthracycline cardiotoxicity: a new paradigm for an old classic. Circulation. 2015;131(22):1946–9.
Middleman E, Luce J, Frei E 3rd. Clinical trials with adriamycin. Cancer. 1971;28(4):844–50.
Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94(4):525–33.
Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–8.
Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23(12):2629–36.
van der Pal HJ, van Dalen EC, Hauptmann M, Kok WE, Caron HN, van den Bos C, et al. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med. 2010;170(14):1247–55.
Ryberg M, Nielsen D, Skovsgaard T, Hansen J, Jensen BV, Dombernowsky P. Epirubicin cardiotoxicity: an analysis of 469 patients with metastatic breast cancer. J Clin Oncol. 1998;16(11):3502–8.
Samuel L, Cummings J, Shaw P. Daunorubicin cardiotoxicity in childhood cancer. Lancet. 1998;352(9134):1150.
Lubieniecka JM, Graham J, Heffner D, Mottus R, Reid R, Hogge D, et al. A discovery study of daunorubicin induced cardiotoxicity in a sample of acute myeloid leukemia patients prioritizes P450 oxidoreductase polymorphisms as a potential risk factor. Front Genet. 2013;4:231.
Lipshultz SE, Lipsitz SR, Kutok JL, Miller TL, Colan SD, Neuberg DS, et al. Impact of hemochromatosis gene mutations on cardiac status in doxorubicin-treated survivors of childhood high-risk leukemia. Cancer. 2013;119(19):3555–62.
Hasinoff BB. The interaction of the cardioprotective agent ICRF-187 ((+)-1,2-bis (3,5-dioxopiperazinyl-1-yL) propane); its hydrolysis product (ICRF-198); and other chelating agents with the Fe (III) and cu (II) complexes of adriamycin. Agents Actions. 1989;26(3–4):378–85.
Hasinoff BB, Patel D, Wu X. The oral iron chelator ICL670A (deferasirox) does not protect myocytes against doxorubicin. Free Radic Biol Med. 2003;35(11):1469–79.
Martin E, Thougaard AV, Grauslund M, Jensen PB, Bjorkling F, Hasinoff BB, et al. Evaluation of the topoisomerase II-inactive bisdioxopiperazine ICRF-161 as a protectant against doxorubicin-induced cardiomyopathy. Toxicology. 2009;255(1–2):72–9.
Sawyer DB. Anthracyclines and heart failure. N Engl J Med. 2013;368(12):1154–6.
Lyu YL, Kerrigan JE, Lin CP, Azarova AM, Tsai YC, Ban Y, et al. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007;67(18):8839–46.
Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35(8):893–911.
Rabinovich A, Weiss D, Weissman M, Novack V, Levi I. Dexrazoxane does not affect treatment outcome in non-Hodgkin’s lymphoma patients. Am J Hematol. 2012;87(8):830–2.
Limat S, Daguindau E, Cahn JY, Nerich V, Brion A, Perrin S, et al. Incidence and risk-factors of CHOP/R-CHOP-related cardiotoxicity in patients with aggressive non-Hodgkin’s lymphoma. J Clin Pharm Ther. 2014;39(2):168–74.
Vachhani P, Shin S, Baron J, Thompson JE, Wetzler M, Griffiths EA, et al. Dexrazoxane for cardioprotection in older adults with acute myeloid leukemia. Leuk Res Rep. 2017;7:36–9.
Cvetkovic RS, Scott LJ. Dexrazoxane: a review of its use for cardioprotection during anthracycline chemotherapy. Drugs. 2005;65(7):1005–24.
Lopez M, Vici P, Di Lauro K, Conti F, Paoletti G, Ferraironi A, et al. Randomized prospective clinical trial of high-dose epirubicin and dexrazoxane in patients with advanced breast cancer and soft tissue sarcomas. J Clin Oncol. 1998;16:86–92.
Schuler MK, Gerdes S, West A, Richter S, Busemann C, Hentschel L, et al. Efficacy and safety of dexrazoxane (DRZ) in sarcoma patients receiving high cumulative doses of anthracycline therapy - a retrospective study including 32 patients. BMC Cancer. 2016;16:619.
Chow EJ, Asselin BL, Schwartz CL, Doody DR, Leisenring WM, Aggarwal S, et al. Late mortality after dexrazoxane treatment: a report from the Children’s oncology group. J Clin Oncol. 2015;33(24):2639–45.
Asselin BL, Devidas M, Chen L, Franco VI, Pullen J, Borowitz MJ, et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: a report of the Children’s oncology group randomized trial pediatric oncology group 9404. J Clin Oncol. 2016;34(8):854–62.
Kim H, Kang HJ, Park KD, Koh KN, Im HJ, Seo JJ, et al. Risk factor analysis for secondary malignancy in dexrazoxane-treated pediatric cancer patients. Cancer Res Treat. 2019;51(1):357–67.
Getz KD, Sung L, Leger K, Alonzo TA, Gerbing RB, Cooper TM, et al. Effect of dexrazoxane on left ventricular function and treatment outcomes in patients with acute myeloid leukemia: A Children’s Oncology Group report. J Clin Oncol. 2018;36 (suppl; abstr 10501).
Reichardt P, Tabone MD, Mora J, Morland B, Jones RL. Risk-benefit of dexrazoxane for preventing anthracycline-related cardiotoxicity: re-evaluating the European labeling. Future Oncol. 2018;14(25):2663–76.
Barrett-Lee PJ, Dixon JM, Farrell C, Jones A, Leonard R, Murray N, et al. Expert opinion on the use of anthracyclines in patients with advanced breast cancer at cardiac risk. Ann Oncol. 2009;20(5):816–27.
Ganatra S, Neilan TG. Immune checkpoint inhibitor associated myocarditis. Oncologist. 2018;23:518–23.
Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–64.
Ganatra S, Sharma A, Shah S, Chaudhry GM, Martin DT, Neilan TG, et al. Ibrutinib-Associated Atrial Fibrillation. JACC Clin Electrophysiol. 2018;4(12):1491–500.
Abdel-Qadir H, Nolan MT, Thavendiranathan P. Routine prophylactic cardioprotective therapy should be given to all recipients at risk of cardiotoxicity from cancer chemotherapy. Can J Cardiol. 2016;32(7):921–5.
Acknowledgements
Not applicable.
Funding
Dr. Lipshultz’ preparation of this article was supported in part by grants from the National Institutes of Health (HL072705, HL078522, HL053392, CA127642, CA068484, HD052104, AI50274, HD52102, HL087708, HL079233, HL004537, HL087000, HL007188, HL094100, HL095127, and HD80002), the National Heart, Lung, and Blood Institute (R01 HL53392, R01 HL111459, R01 HL109090), the Children’s Cardiomyopathy Foundation, the Women’s Cancer Association, the Lance Armstrong Foundation, the STOP Children’s Cancer Foundation, the Parker Family Foundation, the Scott Howard Fund, the Michael Garil Fund, Sofia’s Hope, Inc., and the Kyle John Rymiszewski Foundation.
Dr. Nohria receives research support from Amgen, Inc. and serves as a consultant for Takeda Oncology.
Dr. Zarwan serves as an adviser for Revere Pharmaceuticals and as an independent clinical data reviewer for Perceptive Informatics.
Dr. Neilan serves on a scientific advisory board for Takeda and as a consultant at Parexal.
Availability of data and materials
All the relevant data and material are presented in the main manuscript.
Author information
Authors and Affiliations
Contributions
All authors have contributed sufficiently in the conception and design of the study, data collection and interpretation as well as manuscript preparation. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Lahey Clinic, Inc. IRB has approved the project and waived the requirement for consent.
Consent for publication
Not applicable. (This manuscript does not contain any identifiable individual information and Lahey Clinic, Inc. IRB has waived the requirement for consent).
Competing interests
Dr. Lipshultz is a consultant for Clinigen Group plc, which manufactures dexrazoxane. Dr. Lipshultz has received investigator-initiated research grants from Pharmacia and Upjohn, Chiron Corporation, and Pfizer to study dexrazoxane.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ganatra, S., Nohria, A., Shah, S. et al. Upfront dexrazoxane for the reduction of anthracycline-induced cardiotoxicity in adults with preexisting cardiomyopathy and cancer: a consecutive case series. Cardio-Oncology 5, 1 (2019). https://doi.org/10.1186/s40959-019-0036-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40959-019-0036-7