Summary
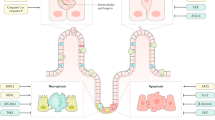
Apoptosis, autophagy and necrosis are three distinct functional types of the mammalian cell death network. All of them are characterized by a number of cell’s morphological changes. The inappropriate induction of cell death is involved in the pathogenesis of a number of diseases.
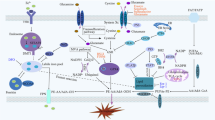
Pathogenesis of inflammatory bowel diseases (ulcerative colitis, Crohn’s disease) includes an abnormal immunological response to disturbed intestinal microflora. One of the most important reason in pathogenesis of chronic inflammatory disease and subsequent multiple organ pathology is a barrier function of the gut, regulating cellular viability. Recent findings have begun to explain the mechanisms by which intestinal epithelial cells are able to survive in such an environment and how loss of normal regulatory processes may lead to inflammatory bowel disease (IBD).
This review focuses on the regulation of biological pathways in development and homeostasis in IBD. Better understanding of the physiological functions of biological pathways and their influence on inflammation, immunity, and barrier function will simplify our expertice of homeostasis in the gastrointestinal tract and in upgrading diagnosis and treatment.
Zusammenfassung
Apoptose, Autophagie und Nekrose sind drei unterschiedliche Funktionsformen des Zelltodes bei Säugetieren. Alle sind durch etliche morphologische Veränderungen in den Zellen gekennzeichnet. Eine fehlerhafte Induktion des Zelltodes ist Teil der Pathogenese vieler Krankheiten.
Die Pathogenese entzündlicher Darmerkrankungen (Colitis ulcerosa, Morbus Crohn) schließt auch eine anormale Immunreaktion auf die gestörte Darmmikroflora ein. Einer der wichtigsten Gründe für die Pathogenese einer chronisch-entzündlichen Krankheit und folglich einer Multiorganpathologie ist die Barrierefunktion des Darms, die für die Regelung der Zellviabilität zuständig ist. Neuere Erkenntnisse helfen zu erklären, welche Mechanismen dazu führen, dass Darmepithelzellen in einem derartigen Umfeld überleben können und wie der Verlust normaler regulativer Prozesse zu entzündlichen Darmerkrankungen führen können.
Dieser Bericht beschäftigt sich mit der Regulierung biologischer Wege in der Entwicklung und Homöostase entzündlicher Darmerkrankungen. Ein besseres Verständnis der physiologischen Funktionen der biologischen Wege und deren Einfluss auf Entzündungen, Immunität und Barrierefunktion wird unser Fachwissen zum Thema Homöostase im Verdauungstrakt verbessern und sowohl Diagnose als auch Behandlung vereinfachen.
Similar content being viewed by others
References
Ayabe T, Satchell DP, Wilson CL, et al. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1(2):113–8.
Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126–30.
Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–48.
O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7(7):688–93.
Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010;28:623–67.
Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4(6):478–85.
McCormick BA, Parkos CA, Colgan SP, Carnes DK, Madara JL. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160(1):455–66.
Kagnoff MF. Microbial-epithelial cell crosstalk during inflammation: the host response. Ann N Y Acad Sci. 2006;1072:313–20.
Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100(1):6–10.
Jess T, Riis L, Vind I, et al. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis. 2007;13(4):481–9.
Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91(4):854–62.
Jess T, Riis L, Jespersgaard C, et al. Disease concordance, zygosity, and NOD2/CARD15 status: follow-up of a population-based cohort of Danish twins with inflammatory bowel disease. Am J Gastroenterol. 2005;100(11):2486–92.
Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474(7351):307–17.
Sartor RB. Intestinal microflora in human and experimental inflammatory bowel disease. Curr Opin Gastroenterol. 2001;17(4):324–30.
Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92(Suppl. 12):5S–11S.
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347(6):417–29.
Farrell RJ, LaMont JT. Microbial factors in inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31(1):41–62.
Shanahan F. Host-flora interactions in inflammatory bowel disease. Inflamm Bowel Dis. 2004;10(Suppl. 1):S16–24.
Swidsinski A, Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122(1):44–54.
Neut C, Bulois P, Desreumaux P, et al. Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn’s disease. Am J Gastroenterol. 2002;97(4):939–46.
Darfeuille-Michaud A, Neut C, Barnich N, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn’s disease. Gastroenterology. 1998;115(6):1405–13.
Seksik P, Sokol H, Lepage P, et al. Review article: the role of bacteria in onset and perpetuation of inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24(Suppl. 3):11–8.
Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004;53(1):1–4.
Barnich N, Darfeuille-Michaud A. Role of bacteria in the etiopathogenesis of inflammatory bowel disease. World J Gastroenterol. 2007;13(42):5571–6.
Gersemann M, Stange EF, Wehkamp J. From intestinal stem cells to inflammatory bowel diseases. World J Gastroenterol. 2011;17(27):3198–203.
Garret WS, Gordon JI, Glimcher LH. Homeostasis and inflamation in the intestine. Cell. 2010;140(6):859–70.
Cadwell K, Stappenbeck TS, Virgin HW. Role of autophagy and autophagy genes in inflammatory bowel disease. Curr Top Microbiol Immunol. 2009;335:141–67.
Deretic V, Delgado M, Vergne I, et al. Autophagy in immunity against mycobacterium tuberculosis: a model system to dissect immunological roles of autophagy. Curr Top Microbiol Immunol. 2009;335:169–88.
Deretic V. Autophagy in innate and adaptive immunity. Trends Immunol. 2005;26(10):523–8.
Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7(10):767–77.
Schmid D, Munz C. Innate and adaptive immunity through autophagy. Immunity. 2007;27(1):11–21.
Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest. 2006;116(9):2366–77.
Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem. 2006;281(16):11374–83.
Checroun C, Wehrly TD, Fischer ER, Hayes SF, Celli J. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc Natl Acad Sci U S A. 2006;103(39):14578–83.
Cullinane M, Gong L, Li X, et al. Stimulation of autophagy suppresses the intracellular survival of Burkholderia pseudomallei in mammalian cell lines. Autophagy. 2008;4(6):744–53.
Gutierrez MG, Master SS, Singh SB, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119(6):753–66.
Liang XH, Kleeman LK, Jiang HH, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72(11):8586–96.
Ling YM, Shaw MH, Ayala C, et al. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203(9):2063–71.
Liu Y, Schiff M, Czymmek K, et al. Autophagy regulates programmed cell death during the plant innate immune response. Cell. 2005;121(4):567–77.
Nakagawa I, Amano A, Mizushima N, et al. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306(5698):1037–40.
Ogawa M, Yoshimori T, Suzuki T, et al. Escape of intracellular Shigella from autophagy. Science. 2005;307(5710):727–31.
Orvedahl A, Alexander D, Talloczy Z, et al. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1(1):23–35.
Py BF, Lipinski MM, Yuan J. Autophagy limits Listeria monocytogenes intracellular growth in the early phase of primary infection. Autophagy. 2007;3(2):117–25.
Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313(5792):1438–41.
Talloczy Z, Jiang W, Virgin HWt, et al. Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc Natl Acad Sci U S A. 2002;99(1):190–5.
Yano T, Mita S, Ohmori H, et al. Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat Immunol. 2008;9(8):908–16.
Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–75.
Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443(7113):780–6.
Iwata J, Ezaki J, Komatsu M, et al. Excess peroxisomes are degraded by autophagic machinery in mammals. J Biol Chem. 2006;281(7):4035–41.
Okamoto K, Kondo-Okamoto N, Ohsumi Y. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev Cell. 2009;17(1):87–97.
Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4(12):e423.
Deretic V. Autophagy in infection. Curr Opin Cell Biol. 2010;22(2):252–62.
Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–93.
Hampe J, Franke A, Rosenstiel P, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39(2):207–11.
Massey DC, Parkes M. Genome-wide association scanning highlights two autophagy genes, ATG16L1 and IRGM, as being significantly associated with Crohn’s disease. Autophagy. 2007;3(6):649–51.
Kuballa P, Huett A, Rioux JD, Daly MJ, Xavier RJ. Impaired autophagy of an intracellular pathogen induced by a Crohn’s disease associated ATG16L1 variant. PLoS One. 2008;3(10):e3391.
Zhang H, Massey D, Tremelling M, Parkes M. Genetics of inflammatory bowel disease: clues to pathogenesis. Br Med Bull. 2008;87:17–30.
Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5(6):527–49.
Lerena C, Calligaris SD, Colombo MI. Autophagy: for better or for worse, in good times or in bad times. Curr Mol Med. 2008;8(2):92–101.
Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9(12):1004–10.
Kim M, Ashida H, Ogawa M, et al. Bacterial interactions with the host epithelium. Cell Host Microbe. 2010;8(1):20–35.
Huang J, Brumell JH. Autophagy in immunity against intracellular bacteria. Curr Top Microbiol Immunol. 2009;335:189–215.
Hubbard VM, Cadwell K. Viruses, autophagy genes, and Crohn’s disease. Viruses. 2011;3(7):1281–311.
Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22(2):124–31.
Yousefi S, Simon HU. Autophagy in cells of the blood. Biochim Biophys Acta. 2009;1793(9):1461–4.
Hockenbery D. Defining apoptosis. Am J Pathol. 1995;146(1):16–9.
Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107(Pt 12):3569–77.
Potten CS. The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev. 1992;11(2):179–95.
Mathan MM, Mathan VI. Morphology of rectal mucosa of patients with shigellosis. Rev Infect Dis. 1991;13(Suppl. 4):S314–8.
Savidge TC, Shmakov AN, Walker-Smith JA, Phillips AD. Epithelial cell proliferation in childhood enteropathies. Gut. 1996;39(2):185–93.
Islam MM, Azad AK, Bardhan PK, Raqib R, Islam D. Pathology of shigellosis and its complications. Histopathology. 1994;24(1):65–71.
Sachdev HP, Chadha V, Malhotra V, Verghese A, Puri RK. Rectal histopathology in endemic Shigella and Salmonella diarrhea. J Pediatr Gastroenterol Nutr. 1993;16(1):33–8.
Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43(7):3380–9.
Pacelli R, Wink DA, Cook JA, et al. Nitric oxide potentiates hydrogen peroxide-induced killing of Escherichia coli. J Exp Med. 1995;182(5):1469–79.
Weersma RK, van Dullemen HM, van der Steege G, et al. Review article: Inflammatory bowel disease and genetics. Aliment Pharmacol Ther. 2007;26(Suppl. 2):57–65.
Wapenaar MC, Monsuur AJ, van Bodegraven AA, et al. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis. Gut. 2008;57(4):463–7.
Strus M, Gosiewski T, Fyderek K, et al. A role of hydrogen peroxide producing commensal bacteria present in colon of adolescents with inflammatory bowel disease in perpetuation of the inflammatory process. J Physiol Pharmacol. 2009;60(Suppl. 6):49–54.
Denning TL, Takaishi H, Crowe SE, et al. Oxidative stress induces the expression of Fas and Fas ligand and apoptosis in murine intestinal epithelial cells. Free Radic Biol Med. 2002;33(12):1641–50.
Kaczmarek M, Frydrychowicz M, Nowicka A, et al. Influence of pleural macrophages on proliferative activity and apoptosis regulating proteins of malignant cells. J Physiol Pharmacol. 2008;59(Suppl. 6):321–30.
Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol. 2003;201(1):28–36.
Kim JM, Eckmann L, Savidge TC, et al. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Invest. 1998;102(10):1815–23.
Jain MV, Paczulla AM, Klonisch T, et al. Interconnections between apoptotic, autophagic and necrotic pathways: implications for cancer therapy development. J Cell Mol Med. 2013;17(1):12–29.
Skulachev VP. Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis. 2006;11(4):473–85.
Los M, Mozoluk M, Ferrari D, et al. Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol Biol Cell. 2002;13(3):978–88.
Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503.
Rahman MM, Lucas AR, McFadden G. Viral TNF inhibitors as potential therapeutics. Adv Exp Med Biol. 2009;666:64–77.
Chouaib S, Robinet E, Zyad A, Branellec D. Tumor necrosis factor: pleiotropic cytokine. Bull Cancer. 1992;79(10):935–49.
Sidhu RS, Bollon AP. Tumor necrosis factor activities and cancer therapy–a perspective. Pharmacol Ther. 1993;57(1):79–128.
Keane J. TNF-blocking agents and tuberculosis: new drugs illuminate an old topic. Rheumatology (Oxford). 2005;44(6):714–20.
Nash PT, Florin TH. Tumour necrosis factor inhibitors. Med J Aust. 2005;183(4):205–8.
Zariffard MR, Novak RM, Lurain N, et al. Induction of tumor necrosis factor- alpha secretion and toll-like receptor 2 and 4 mRNA expression by genital mucosal fluids from women with bacterial vaginosis. J Infect Dis. 2005;191(11):1913–21.
Wang J, Barke RA, Charboneau R, Roy S. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J Immunol. 2005;174(1):426–34.
Turner JD, Langley RS, Johnston KL, et al. Wolbachia endosymbiotic bacteria of Brugia malayi mediate macrophage tolerance to TLR- and CD40-specific stimuli in a MyD88/TLR2-dependent manner. J Immunol. 2006;177(2):1240–9.
Corredor J, Yan F, Shen CC, et al. Tumor necrosis factor regulates intestinal epithelial cell migration by receptor-dependent mechanisms. Am J Physiol Cell Physiol. 2003;284(4):C953–61.
Plevy SE, Landers CJ, Prehn J, et al. A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn's disease. J Immunol. 1997; 159(12):6276–82.
Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337(15):1029–35.
Saito M, Katsuno T, Nakagawa T, et al. Intestinal epithelial cells with impaired autophagy lose their adhesive capacity in the presence of TNF-alpha. Dig Dis Sci. 2012;57(8):2022–30.
Holler N, Zaru R, Micheau O, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1(6):489–95.
Vercammen D, Vandenabeele P, Beyaert R, Declercq W, Fiers W. Tumour necrosis factor-induced necrosis versus anti-Fas-induced apoptosis in L929 cells. Cytokine. 1997;9(11):801–8.
Gunther C, Martini E, Wittkopf N, et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477(7364):335–9.
Pastorelli L, De Salvo C, Mercado JR, Vecchi M, Pizarro TT. Central Role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front Immunol. 2013;4:280.
Ardizzone S, Bianchi Porro G. Biologic therapy for inflammatory bowel disease. Drugs. 2005;65(16):2253–86.
Suenaert P, Bulteel V, Lemmens L, et al. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am J Gastroenterol. 2002;97(8):2000–4.
Suenaert P, Bulteel V, Vermeire S, et al. Hyperresponsiveness of the mucosal barrier in Crohn’s disease is not tumor necrosis factor-dependent. Inflamm Bowel Dis. 2005;11(7):667–73.
Mirpuri J, Brazil JC, Berardinelli AJ, et al. Commensal Escherichia coli reduces epithelial apoptosis through IFN-alphaA-mediated induction of guanylate binding protein-1 in human and murine models of developing intestine. J Immunol. 2010;184(12):7186–95.
Ivanov, II, Frutos Rde L, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–49.
Onizawa M, Nagaishi T, Kanai T, et al. Signaling pathway via TNF-alpha/NF-kappaB in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2009;296(4):G850–9.
Kanai T, Totsuka T, Uraushihara K, et al. Blockade of B7-H1 suppresses the development of chronic intestinal inflammation. J Immunol. 2003;171(8):4156–63.
MacDonald TT, Monteleone G, Pender SL. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000;51(1):2–9.
Totsuka T, Kanai T, Iiyama R, et al. Ameliorating effect of anti-inducible costimulator monoclonal antibody in a murine model of chronic colitis. Gastroenterology. 2003;124(2):410–21.
Lapaquette P, Brest P, Hofman P, Darfeuille-Michaud A. Etiology of Crohn’s disease: many roads lead to autophagy. J Mol Med (Berl). 2012;90(9):987–96.
Cadwell K, Patel KK, Maloney NS, et al. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell. 2010;141(7):1135–45.
Rutgeerts P, Goboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet. 1991;338(8770):771–4.
Man SM, Kaakoush NO, Mitchell HM. The role of bacteria and pattern-recognition receptors in Crohn’s disease. Nat Rev Gastroenterol Hepatol. 2011;8(3):152–68.
Pagnini C, Cominelli F. Probiotics in experimental and human inflammatory bowel disease: discussion points. Dig Liver Dis. 2006;38(Suppl. 2):S270–3.
Vanderpool C, Yan F, Polk DB. Mechanisms of probiotic action: Implications for therapeutic applications in inflammatory bowel diseases. Inflamm Bowel Dis. 2008;14(11):1585–96.
Bai AP, Ouyang Q. Probiotics and inflammatory bowel diseases. Postgrad med J. 2006;82(968):376–82.
Bernet MF, Brassart D, Neeser JR, Servin AL. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35(4):483–9.
Schultz M, Lindstrom AL. Rationale for probiotic treatment strategies in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2008;2(3):337–55.
Maddika S, Ande SR, Panigrahi S, et al. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Updat. 2007;10(1–2):13–29.
Chaabane W, User SD, El-Gazzah M, et al. Autophagy, apoptosis, mitoptosis and necrosis: interdependence between those pathways and effects on cancer. Arch Immunol Ther Exp (Warsz). 2013;61(1):43–58.
Stroh C, Cassens U, Samraj A, et al. The role of caspases in cryoinjury: caspase inhibition strongly improves the recovery of cryopreserved hematopoietic and other cells. FASEB J. 2002;16(12):1651–3.
Alavian SM, Ande SR, Coombs KM, et al. Virus-triggered autophagy in viral hepatitis—possible novel strategies for drug development. J Viral Hepat. 2011;18(12):821–30.
Panigrahi S, Stetefeld J, Jangamreddy JR, et al. Modeling of molecular interaction between apoptin, BCR-Abl and CrkL–an alternative approach to conventional rational drug design. PLoS One. 2012;7(1):e28395.
Conflict of interest
The authors declare that there is no actual or potential conflict of interest in relation with this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zemljic, M., Pejkovic, B., Krajnc, I. et al. Biological pathways involved in the development of inflammatory bowel disease. Wien Klin Wochenschr 126, 626–633 (2014). https://doi.org/10.1007/s00508-014-0592-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-014-0592-7