Abstract
The last decades have seen extensive scientific and technological improvements in many fields of microbiology and molecular biology. Correspondingly, flow cytometry—a rapid, precise and straightforward method for cultivation-independent detection of cells in liquids—has been a major topic in aquatic microbiology and drinking water analysis. Flow cytometry provides information at the single-cell level, including total cell counts, size measurements, nucleic acid content and bacterial viability and activity. While regulatory requirements for water testing rely on cultivation-based methods, flow cytometry can be considered a powerful tool to complement standard procedures.
This article provides insights into the methodology and applicability of flow cytometry in the field of microbiological drinking water analysis and presents an overview on several case studies that cover a broad range of different objectives. The later are comprised of a study on flow cytometric characterization of Austrian drinking water resources, of an example for advanced data analysis methods of flow cytometric data, of a study on monitoring microbial regrowth within the distribution network, of an exemplary case of the application of online flow cytometry for high-frequency monitoring and of an introduction to the combination of flow cytometry and sequencing information.
Finally, it is argued that due to the high microbiological variability of different water resources, unusual changes of flow cytometric parameters, rather than specific limits, could act as an indicator for further investigation. In this way flow cytometry can provide a good basis for risk assessments in water safety plans. The application of flow cytometry still remains utility-specific and a huge need for standardization of data analysis and interpretation exists in order to achieve a better cooperation of water utilities.
Zusammenfassung
Die letzten Jahrzehnte haben in vielen Bereichen der Mikrobiologie und Molekularbiologie umfangreiche wissenschaftliche und technologische Fortschritte gebracht. Dementsprechend ist die Durchflusszytometrie – eine schnelle, präzise und unkomplizierte Methode zum kultivierungsunabhängigen Nachweis von Zellen in Flüssigkeiten – ein wichtiges Thema in der aquatischen Mikrobiologie und der Trinkwasseranalyse geworden. Die Durchflusszytometrie liefert Informationen auf Ebene einzelner Zellen und erlaubt dabei Größenbestimmungen, Messungen des Gehalts an Nukleinsäuren, Aspekte über bakterielle Lebensfähigkeit und Aktivität sowie die Ermittlung von Gesamtzellzahlen. Während regulatorische Anforderungen für die Trinkwasseruntersuchung auf kultivierungsbasierte Methoden ausgelegt sind, kann die Durchflusszytometrie als leistungsstarke Ergänzung von Standardverfahren angesehen werden.
Dieser Artikel gibt Einblicke in die Methodik und Anwendbarkeit der Durchflusszytometrie im Bereich der mikrobiologischen Trinkwasseranalyse und stellt mehrere Fallstudien vor, die ein breites Spektrum unterschiedlicher Zielsetzungen abdecken. Letztere umfassen eine Studie zur durchflusszytometrischen Charakterisierung österreichischer Trinkwasserressourcen, ein Beispiel für fortgeschrittene Datenanalysemethoden durchflusszytometrischer Daten, eine Studie zur Überwachung der mikrobiellen Dynamik im Verteilungsnetz, einen exemplarischen Fall der Anwendung von Online-Durchflusszytometrie für ein verdichtetes Monitoring und eine Einführung in die Kombination von Durchflusszytometrie und Sequenzierungsverfahren.
Abschließend wird argumentiert, dass aufgrund der hohen mikrobiologischen Variabilität verschiedener Wasserressourcen ungewöhnliche Veränderungen von durchflusszytometrischen Parametern als Anlass für weitere Untersuchungen dienen sollten. Auf diese Weise kann die Durchflusszytometrie eine ergänzende Grundlage für die Risikobewertung im Rahmen der Erstellung von Wassersicherheitsplänen darstellen. Die Anwendung der Durchflusszytometrie ist nach wie vor nutzungs- bzw. betriebsspezifisch und es besteht ein großer Bedarf an einer Standardisierung der Datenanalyse und -interpretation, um eine bessere Zusammenarbeit von Wasserversorgern zu erreichen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Flow cytometry (FC) refers to the analysis of suspended particles based on scattered light and/or fluorescence when passing a laser beam. The development of FC started in the 1960‘s with first applications in the medical field. Due to technical limitations mainly related to the small size of bacterial cells its implementation did not immediately become popular in the field of environmental microbiology (Wang et al. 2010) (Egli and Kötzsch 2015). The last decades have seen notable technological improvements and a huge increase in research and successful applications of FC in aquatic microbiology and drinking water analysis. While regulatory requirements for water testing rely on cultivation-based methods, FC can be considered a powerful tool to complement standard procedures (Safford and Bischel 2019).
There are numerous potentials in the use of FC as a microbiological monitoring technique. Recent studies highlighted FC as a rapid, precise and straightforward method for detecting changes and characterizing the bacterial community in water samples (Hammes, Berney, and Egli 2010; Prest et al. 2013; Gillespie et al. 2014; Van Nevel et al. 2017; Safford and Bischel 2019). It can provide a broad range of information at the single-cell level, including total cell counts, size measurements, nucleic acid content and aspects in bacterial viability and activity (Hammes, Berney, and Egli 2010; Prest et al. 2013). Here, the term total cell count indicates the possibility to receive a good approximation of the true number of bacteria or cells in a sample volume, including viable, non-viable and viable but nonculturable cells. The later property might be seen as the most distinguishing feature as compared to standard culture-based methods, that rely on bacterial proliferation in a more or less selective environment (Van Nevel et al. 2017). While no regulatory limits for drinking water have been set so far, quantification of total bacterial numbers can give important additional information not only to the understanding of microbial survival and growth during drinking water treatment and distribution (Hammes et al. 2008) but also to monitoring source water quality in high temporal resolution (Besmer and Hammes 2016).
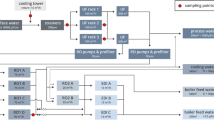
The measurement principle of FC relies on the interaction between the laser beam and the suspended particle that causes scattering of light and excitation of fluorochromes (Fig. 1). The latter are added to the sample prior to the FC measurement and bind specifically to different target regions within cells. The scattered light and emitted fluorescent light are detected and measured with photomultipliers and wavelength filters. Due to the electronic signal detection up to at least 1000 particles per second can be counted, with generally less than a milliliter of sample volume. This automatized nature of the detection process can be seen as a major difference to a further fluorescence-based method, i.e. epifluorescence microscopy. While allowing for a high degree of flexibility in application and a more visually guided workflow, obtaining bacterial counts is time-consuming even if automated image analysis systems are employed (Felip et al. 2007). Nonetheless, epifluorescence microscopy serves as a first choice and important reference standard if comparative measurements to FC are needed. FC signals of cells are digitalized data of the fluorescence light intensity and are typically presented as single parameter histograms or dual-parameter dot plots.
Principles of flow cytometry analysis adapted from (Egli 2010)
The main advantages of FC are rapidity with 10 min staining time and < 1 min analysis time (Van Nevel et al. 2017), accuracy with less than 3%, 5% and 15% relative standard deviation of repeated measurements according to different authors (Hammes et al. 2008; Gillespie et al. 2014), reproducibility with < 7% variability between different laboratories (Van Nevel et al. 2017) and sensitivity with a detection limit as low as 32, 100 and 200 cells per milliliter according to different sources (Gillespie et al. 2014; SLMB 2012; Hammes et al. 2008). While this performance features are very well suited to measure abundant total cell counts in drinking water applications, FC might not be the method of choice for the detection of specific organisms of low (using particularly tailored probes) that occur in very low abundances (e.g. one per 100 milliliters).
The aim of this article is to provide insights into the methodology and applicability of FC in the field of microbiological drinking water analysis. To this end several case studies are presented that cover a broad range of different objectives relevant to the practitioner as well as scientific research.
2 Methodology
FC heavily relies on the preparation of the sample and especially the application of fluorescent dyes that specifically target certain compartments of cells. It is essential for FC that not only quantitative information (TCC) can be derived, but also qualitative information about the respective cells. Application of a nucleic acid dye like SYBR Green 1 targets the nucleic acids within a cell and the strength of the fluorescence signal depends on the amount of nucleic acid. In this context, the typical occurrence of at least two well distinguishable groups has been described in the literature of aquatic microbiology (Lebaron et al. 2001; Wang et al. 2009; Proctor et al. 2018). Accordingly, these are designated as cells with low (LNA) and high (HNA) nucleic acid content. Fig. 2 shows a typical measurement result of a groundwater sample as a scatter plot of the signal combination of green and red fluorescence together with so-called gates. The latter serve to delimit certain signal ranges in the course of data analysis, e.g. to separate cell signals from the signal background. Various fluorescence dyes exist to measure different qualities of cells, e.g. CFDA for enzymatic activity. Most notably, the use of SYBR Green 1 together with propidium iodide for the assessment of cellular membrane damage is widely used (Stocks 2004). The respective parameter is called intact cell count (ICC), i.e. the concentration of cells with intact cell membranes.
Ideally, identical samples should lead to identical signal intensities. However, there are various reasons that easily cause deviations from this notion, e.g. differences between flow cytometers (even for the same type of model) or small variabilities in incubation times and temperatures of the dye (Van Nevel et al. 2013). Though generally considered a robust method, it is of paramount importance to establish measures for quality assurance for FC measurements. Data analysis is commonly based on the usage of fixed gates for different samples that originate from subjective decisions of the FC specialist (Prest et al. 2013). This would be advantageous if differences in signal intensities represent true biological information, but less so if those differences originate from other factors like sample preparation. A data-driven approach to establish automatic gating procedures could be beneficial in such situations (García, López-Urrutia, and Morán 2014). Moreover, such procedures could aid in establishing unified operational standards since no or very little subjective human judgement is involved. A striking example for these kinds of difficulties is presented in Fig. 3. Separation of LNA and HNA should rely on the density distributions of fluorescent signals. The mere adoption of templates from other operators (green line) fails to correctly separate the two, while automatized procedures (blue line) are able to capture the data structure (Zunabovic-Pichler et al. 2018).
Details of the methodology for sample handling and flow cytometric measurements used in the case studies are given in Fiedler et al. (2018) and Zunabovic-Pichler et al. (2018). In short, 50 ml of sample were collected in sterile centrifuge tubes (VWR, United States), kept cool at 4 °C and processed within 48 h. For the measurement 297 µl of well mixed sample were either stained with 3 µl of SYBR Green I (Life Technologies, Eugene, OR, United States) diluted 1:100 with DMSO (Fluka, Switzerland) for TCC or with a mixture of SYBR Green I and propidium iodide (6 µM final concentration) diluted 1:100 with 10 mM Tris buffer (pH = 8.1) for ICC. The sample was subsequently incubated in the dark for 13 min. Flow cytometric measurements were performed using a BD Accuri C6 flow cytometer (BD Life Sciences, CA, United States) containing a 50 mW laser emitting at a wavelength of 488 nm. Measurement volume was set to 50 µl, flow rate to 35 µl/min. Measurements were performed in triplicates. Data were processed using the R package flowCore. Bacterial signals were differentiated from background noise by electronic gating using a fixed gate approach. All cell counts are given as particles per 1 ml.
Specific details on e.g. sampling locations are provided within the presentation of the case study at hand.
3 Presentation of case studies
3.1 Case study 1: characterization of Austrian water resources by FC-TCC
As major part of the study “Durchflusszytometrie in der Wasserwirtschaft” (Zunabovic-Pichler et al. 2018), that assessed the potential of FC for Austrian water supplies, a measurement campaign covering raw and drinking water samples from 58 sampling locations (provinces: Upper and Lower Austria, Burgenland and Vienna) with different water resources was conducted. Samples were collected weekly or biweekly in the course of one year. This campaign represents the first comprehensive investigation of the state of Austrian water resources using FC.
Fig. 4 summarizes the TCC and the HNA ratios of different sampling locations and resources. The black line represents the median of the individual measurement series, the box itself indicates the 25% and 75% quantiles. With a few exceptions, monitoring sites in pore groundwaters, springs, and deep groundwaters show lower and often very constant cell counts. Karst springs and bank filtrates have higher cell and often show a greater variability. The lowest HNA ratios could be detected in the group of springs. The group of bank filtrates show a very constant HNA ratio of just under 50% for almost all measuring points. Some of the pore groundwaters and especially the deep groundwaters represent measuring points with the highest HNA proportions.
The obtained numbers for the TCC in Austrian are similar to those obtained for Swiss groundwaters (Kötzsch and Sinreich 2014), but as to the cause of these patterns no specific answer can be provided other than that there is likely a close relationship of TCC and the availability of dissolved organic carbon and nutrients (Griebler and Lueders 2009). HNA fractions are indicative of microbial community structure with small HNA fractions (i.e. large LNA fractions) pointing towards bacteria with reduced genomes and size (Proctor et al. 2018). It remains a promising topic for the future to illuminate the role of certain groups in the respective environments leading to a better understanding of the microbial dynamics of groundwater resources.
3.2 Case study 2: flow cytometric fingerprints—capturing all information
Cytometric fingerprinting is a data analysis method that offers the possibility to compare the (multidimensional) signal structure of different FC measurements in great detail (Rogers et al. 2008; Koch et al. 2013; Buysschaert et al. 2018). The method exploits the fact that individual detections of a FC measurement can show very different signal intensities on several measurement channels. These differences can be associated with certain properties of the particles (e.g. the differentiation between LNA and HNA cells). Based on the individual signal characteristics groups can be defined which can be used for further analysis or comparison with other data sources, e.g. typical water monitoring parameters (electrical conductivity, dissolved organic carbon, SAK at 254 nm, etc.).
From a methodological point of view cytometric fingerprinting is essentially based on the application of a counting grid adapted to the density of detections within the selected signal combinations (Fig. 5). The individual grid cells (“bins”) are supposed to contain the same number of data points on average across all data sets to be compared. The data sets are then counted using this grid which results in a one-dimensional representation of the data. If the considered data sets differ, this counting will result in deviations from the average value or different counting results will occur in one or more bins. At the same time, the data areas in which these differences occur can also be identified.
Cytometric fingerprinting typically shows that measurements with a high number of cell detections do not necessarily differ with respect to the distribution of signal intensities from measurements of the same measuring point, which have considerably less cell detections. In contrast, different water resources can regularly be distinguished very clearly. Another application is the detection of outliers in a data set that includes a large number of measurements—as is the case with the online FC. The potential outliers correspond to “rare” groups, whose signal structure—but not necessarily their number of detections—deviates strongly from the average.
Within the previously mentioned study “Durchflusszytometrie in der Wasserwirtschaft” cytometric fingerprinting was performed for a total of 2043 measurements of different measuring points focusing on bank filtrate and karst spring water and color-coded in a multidimensional scaling plot (Fig. 6). The latter tries to arrange the data points according to their similarity to each other (the closer, the more similar). For approximately 80% of the values a good separation of the two resource types can be achieved, thus testifying for the increased sensitivity of the approach. It is especially worth mentioning that for different events like flood events (bank filtrate) or heavy rainfall (karst springs) a significant deviation can be found in the fingerprints of the single groups. Cytometric fingerprinting therefore not only helps to distinguish between individual raw waters but can also be used in the practical operation of the FC to identify outliers (or deviations from a defined normal state).
Display of fingerprint distances (distance measure Bray-Curtis) using multidimensional scaling for FC measurements. Color-coded are two large groups within the defined resource types (grey points depict all measurements). The plotted ellipses are based on a fit of a normal distribution and cover 80% of the data points
3.3 Case study 3: monitoring microbial regrowth from resource to tap
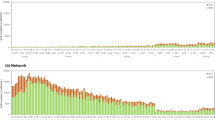
Drinking water distribution networks are complex and highly sensitive systems. Even minor (almost undetectable) contaminations in combination with prolonged residence times can lead to microbial regrowth in the system with consequences for water quality (Wielen and Kooij 2013). Tracing problems within the network can be challenging and sensitive methods are needed (Liu et al. 2018). Since FC is a very sensitive high-throughput methodology it is ideally suited for tracing microbial regrowth in the distribution network given an accurate knowledge of the system’s hydraulics and has been used for this purpose (Schleich et al. 2019; Gabrielli et al. 2021). Fig. 7 shows FC results (ICC) of a growth potential testing series (storing samples under defined conditions) at three locations in a water supply network: immediately after disinfection (combination of chlorine dioxide and UV), outlet of reservoir and tap of customer. A single resource (river bank filtrate) is feeding the network. No or only minor bacterial proliferation could be detected for the sampling location immediately after the disinfection measure, while significant growth could be detected at the reservoir and, to a lesser degree, at the consumer’s tap (storage days 3 and 7 in Fig. 7). It thus appears that a biologically stable water (i.e. no undesired changes in microbiology) is only achieved at the feeding point and that noticeable changes to the water quality can be observed within the network. After close inspection, the cause of the regrowth could be attributed to damages of the coating within the reservoir.
Not only does FC help identify a possible source of contamination, but Fig. 7 also gives testimony to the high sensitivity of method. Comparing measurements from the day of sampling, the sample from the consumers tap shows characteristic similarities to the sample from the reservoir within the region of high signal intensities (HNA cells). Although further in the distribution network the finding of less HNA cells and less pronounced regrowth at the consumer’s tap should be related to the dynamic hydraulic nature of the distribution network, i.e. the reservoir has a common feed and discharge pipe. This leads to situations of possible mixing of water from the reservoir (with longer residence time and possibly contaminated) with fresh water directly from the water treatment plant. This phenomenon of increased HNA cell counts could also be observed at further sampling points within the distribution network as well as at different days.
This case highlights the strength of FC for monitoring distribution networks in regard of biological stability. Already minor biological changes can be made visible with high certainty. The potential to easily process a lot of samples aids at creating a snapshot of the microbial state of a whole distribution network.
3.4 Case study 4: online flow cytometry for high-frequency TCC monitoring
The use of online monitoring methods is state of the art for many water utilities. However, available monitoring systems have mostly recorded physical or chemical parameters so far. A reliable measuring system for on-site use that could record the microbiological state of water at short intervals was missing. Various technical approaches have been proposed and tested (Højris and Skovhus 2018; Lackner et al. 2017), e.g. methods based on enzymatic activity (Demeter et al. 2020), adenosine triphosphate content (de Vera and Wert 2019) or 3D image recognition (Højris et al. 2016). Only a couple of years ago online flow cytometers became available which have since proven to meet the complex requirements for application in different drinking water supply systems (Hammes et al. 2012). For example, the Swiss companies Metanor (Online Bacteria Analyzer) and Sigrist (BactoSense) succeeded in integrating all the necessary measurement steps into a mobile and autonomous device. An add-on device enabling benchtop FC to continuously perform measurements is provided by OnCyt (Switzerland). Various studies demonstrate the application of online flow cytometry for drinking water supply. Microbiological online monitoring can be used to detect changes in raw water at an early stage (Besmer et al. 2014; Sadler et al. 2020) or to track the kinetics of cell damage during various treatment and disinfection processes (Besmer and Hammes 2016; Huang et al. 2016; Adomat et al. 2020; Vergine et al. 2020).
While the combination of grab samples and FC is especially interesting to get a spatial distributed picture of microbial state with drinking water distribution networks, online flow cytometry clearly improves our understanding of the microbial dynamics through high-frequent measurements at certain locations. For the application at hand online flow cytometry (Online Bacteria Analyzer, Metanor) was employed for the study of the temporal variations of a karst spring used for drinking water purposes. Fig. 8 shows the course of the TCC for the period of two months and includes a detailed comparison with FC reference measurements in the laboratory (red dots).
A very good agreement with reference measurements could be achieved which clearly demonstrates the general robustness of the measurement method for determining the TCC. Neither the influence of transport and storage of the samples for the measurements in the laboratory, nor the slightly different sample preparation and especially the different construction and operation of the instruments are influential enough to cause significant differences in the results. For the sampling location in question the highly variable course of the TCC indicates that a sufficiently accurate picture of the microbial state might not be achieved based on daily grab sampling. Short-term peaks with significantly more than 200,000 cells per milliliter are plausible and can also be compared with simultaneously occurring peaks of the SAC at 254 nm, as shown Fig. 9. This could be very useful information regarding the understanding and assessment of possible disinfection measures since problems could arise from high microbial loads in the raw water. Simultaneous measurements of treated water, especially using a viability staining, should significantly add to this process understanding (Besmer et al. 2017; McEvoy et al. 2021).
In the specific case of this sampling location, the correlation of the TCC with the SAC at 254 nm is very striking. This still holds true when the “artificially” generated jumps due to mixing of different springs (rapid change in water composition) are taken into account. The cause of this phenomenon might be seen in the hydraulic nature of karst springs, e.g. with rainfalls mobilizing not only compounds rich in organic carbon but bacteria as well (Besmer et al. 2017). Additionally, it could be argued that higher concentrations of organic substances can sustain higher cell counts. This case thus shows the advantages of high-frequency microbial monitoring especially for highly dynamic water resources like karst springs. The possibility to identify surrogate parameters like the SAC at 254 nm could limit the time of application of online flow cytometry and offer the opportunity to monitor and manage water quality based on more easily available parameters.
Still, a few challenges remain for the broad use of online FC systems. Depending on the design, the system may be more or less “plug and play”, but e.g. the optimal settings of automatic washing cycles (self-cleaning of the instrument) or suitable input tubes to avoid microbial contaminations (biofilms) have to be considered. Testing of optimal settings at the point of use in combination with methodological experience is a strong prerequisite of successful operation.
3.5 Case study 5: combination of flow cytometry and sequencing information
The combination of FC data with microbial community analysis based on High-throughput-sequencing (HTS) might provide additional insights that neither of the methods might bring on their own. Community analysis based on DNA-sequencing solely, will always end with relative abundances of the detected microbial community, whereas merging relative abundances with FC data allows for the calculation of estimated absolute abundances (Fiedler et al. 2018). Otherwise, sequencing data might shed light onto clusters appearing or changing in FC plots. Separation of HNA and LNA-cells by filtration, and downstream microbial community analysis shows that there are taxonomic units that might be found exclusively in one of the clusters (Proctor et al. 2018). An observation based on these findings is presented below.
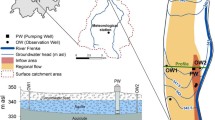
For a small rural Austrian water supplier, a total of 10 samples were taken in January and July 2019. Samples were taken from the only extraction well in use, a following storage tank, and three customers located at the end points of the supply system—each subsequent to the storage tank. Each of the samples was processed for FC analysis as described above and prepared for DNA sequencing. Therefore, 5 l of sample were filtered on 0.2 µm filters, the V3–V4 region of the 16S rRNA gene was amplified by PCR and subsequently sequenced using the Illumina MiSeq platform. After filtering using DADA2 and taxonomic assignment using the SILVA database, relative proportions of sequence variants were projected on FC cell counts, providing estimated absolute abundances which are pictured for the phylum level in Fig. 10.
Results show that the biggest shift in microbial communities occurs for both seasons after extraction from the well within the distribution system. Cluster analysis (not shown) reveals that the microbial communities in the samples from the resource are very stable, with another cluster for both seasonal samples from the storage tank. Interestingly the samples from the customer sites cluster rather by sampling date than location, indicating a seasonal shift in these microbial communities. In winter, the phylum Planctomycetes dominates the samples at the terminal sampling sites, and the total cell counts increase only slightly. Otherwise, in summer, the high increase in total cell counts is attributed to a strong increase in the phylum Patescibacteria. This phylum, which is going through a lot of discussions regarding taxonomy, is consisting of former candidate phyla Parcubacteria, Microgenomates and Gracilibacteria (Rinke et al. 2013). These bacteria are commonly found in groundwater and seem to share properties like reduced genomes, partly symbiotic lifestyles, and very small cell (Tian et al. 2020).
The scatter plot of the FC measurements taken in January and July are shown in Fig. 11. Additionally, to the increase of cells, the ratio of HNA to LNA cells decreases (for r1 from 50% down to 33%), thus showing an overall increase of LNA cells for the customer samples taken in summer.
This increase in LNA cells, which are linked to small size (Wang et al. 2009). In the customer samples, leads to the conclusion that there is a connection to the increase in the estimated absolute abundance of the phylum Patescibacteria in the same samples, as they are known for their small sizes too (Tian et al. 2020). This example clearly supports the idea of a strong connection between FC clusters and taxonomy, and that changes in these clusters are an indicator for changes in the composition of the underlying microbial communities.
4 Summary and outlook
The microbiological analysis of water and drinking water mainly relies on culture-based parameters to ensure safe and biological stable water. The absolute amount and dynamics of ubiquitous bacterial communities is known to be much higher than colony count results defined in various standards. Additionally, a biofilm as main habitat for bacteria is not captured by culture-based methods. Suspended bacteria and particles do not completely represent the ubiquitous microflora in water samples. Nevertheless, FC provides a range of information at the single-cell level, including total cell counts, size measurements, nucleic acid content and bacterial viability and activity (Hammes, Berney, and Egli 2010; Prest et al. 2013). As the sensitivity of FC is variable, relative changes of cell counts are more conclusive than absolute numbers for a microbiological characterization (Prest et al. 2013). The information generated by FC gives added value in the drinking water sector, such as monitoring community changes in water treatment and distribution bypassing the cultured fraction of heterotrophic plate count (Van Nevel et al. 2017). Due to the very low culturability of the water microbiome, HPC counts do not correlate with FC data. It is not intended to compare heterotrophic plate counts with flowcytometric results as both provide different information content. Flowcytometric data give an overview on the current microbiological status in relation to prior data analysed (abnormal events).
Risk assessments in water safety plans need to ensure the understanding of potential risks at process level. Bacterial cell reductions across treatment processes demonstrated a rapid diagnosis on barrier efficiency. Due to the high variability of different water resources, unusual changes of FC parameters, rather than specific limits, should act as an indicator for further investigation. Hence, FC can provide a good basis for risk assessments in water safety plans (Cheswick et al. 2019). The revised Drinking Water Directive foresees the preventive concept by introducing the “risk based approach” (Directive (EU) 2020/2184 2020). Innovative methodologies, such as FC may also serve as important microbiological tool for in-depth analysis in water cycles.
The above mentioned case studies give a brief overview on different applications of FC in combination with microbiological methods and bioinformatics. FC shows high potential to characterize various Austrian drinking water sources regarding total cell counts including subfractions (HNA and LNA) through (event based) fingerprinting over biological stability in water distribution up to combined community analysis results. Bacterial activity, growth potential and community shifts visualized by FC (e.g. HNA/LNA ratio) combined with other methodologies inform on site-specific water quality and its estimated potential risks. Hence, decision making after analysis could enable water utilities to gain confidence in their ongoing processes. From a microbiological point of view problems in drinking water may occur from biostability-associated bacteria, including potential pathogens. This occurrence can be additionally clarified by flowcytometric methodologies. However, the fecal-associated contamination and its indicator bacteria cannot be elucidated through FC analysis. Quantitative flowcytometric microbial risk assessment by targeting waterborne pathogens through tailored probes may be relevant in the context of biological stability and waterborne microbiological dynamics (Hassard and Whitton 2019).
We can expect future FC applications incorporating a series of technologies, such as high-resolution multispectral cell-imaging, MALDI (Matrix-assisted Laser Desorption Ionization) or HTS (High Throughput Sequencing). Also, microfluidic devices might emerge for rapid, low-cost and handheld analysis of bacterial cells in water samples. From an operational perspective, FC is currently seen as an investigative tool for identification of process deviations/water quality events. Standardization of FC remains utility-specific (e.g. gating). However, there is a huge need for data analysis and interpretation standardization in order to achieve a better water utility cooperation challenged by microbiological compliance events.
References
Adomat, Yasmin, Gerit-Hartmut Orzechowski, Marc Pelger, Robert Haas, Rico Bartak, Zsuzsanna Ágnes Nagy-Kovács, Joep Appels, and Thomas Grischek. (2020): New Methods for Microbiological Monitoring at Riverbank Filtration Sites. Water 12 (2): 584.
Besmer M. D., and Hammes F. (2016): Short-Term Microbial Dynamics in a Drinking Water Plant Treating Groundwater with Occasional High Microbial Loads. Water Research 107 (Supplement C): 11–18. https://doi.org/10.1016/j.watres.2016.10.041.
Besmer M. D., Sigrist J. A., Props R., Buysschaert B., Mao G., Boon N., and Hammes F. (2017): Laboratory-Scale Simulation and Real-Time Tracking of a Microbial Contamination Event and Subsequent Shock-Chlorination in Drinking Water. Frontiers in Microbiology 8. https://doi.org/10.3389/fmicb.2017.01900.
Besmer M. D., Weissbrodt D. G., Kratochvil B. E., Sigrist J. A., Weyland M. S., and Hammes F. (2014): The Feasibility of Automated Online Flow Cytometry for In-Situ Monitoring of Microbial Dynamics in Aquatic Ecosystems. Systems Microbiology 5: 265. https://doi.org/10.3389/fmicb.2014.00265.
Buysschaert, Benjamin, Frederiek-Maarten Kerckhof, Peter Vandamme, Bernard De Baets, and Nico Boon. (2018): Flow Cytometric Fingerprinting for Microbial Strain Discrimination and Physiological Characterization. Cytometry Part A 93 (2): 201–12.
Cheswick, Ryan, Elise Cartmell, Susan Lee, Andrew Upton, Paul Weir, Graeme Moore, Andreas Nocker, Bruce Jefferson, and Peter Jarvis. (2019): Comparing Flow Cytometry with Culture-Based Methods for Microbial Monitoring and as a Diagnostic Tool for Assessing Drinking Water Treatment Processes. Environment International 130 (September): 104893.
Demeter K., Burnet J.-B., Stadler P., Kirschner A., Zessner M., and Farnleitner A. H. (2020): Automated Online Monitoring of Fecal Pollution in Water by Enzymatic Methods. Current Opinion in Environmental Science & Health, Occupational safety and health: Emerging Microbial Contaminants and Human Health effects, 16 (August): 82–91. https://doi.org/10.1016/j.coesh.2020.03.002.
Directive (EU) 2020/2184 (2020): Directive (EU) 2020/2184of the European Parliament and of the Council on the Quality of Water Intended for Human Consumption. Official Journal of the European Union. 63 (L 435): 1.
Egli, Thomas. (2010): Neue Methoden Für Die Wasseranalytik. Gas, Wasser, Abwasser : Gwa, no. 90: 315–25.
Egli, Thomas, and Stefan Kötzsch. (2015): Flow Cytometry for Rapid Microbiological Analysis of Drinking Water: From Science to Practice, an Unfinished Story. In Flow Cytometry in Microbiology: Technology and Applications, 175–216. Caister Academic Press.
Felip M., Andreatta S., Sommaruga R., Straskrábová V., and Catalan J. (2007): Suitability of Flow Cytometry for Estimating Bacterial Biovolume in Natural Plankton Samples: Comparison with Microscopy Data. Applied and Environmental Microbiology 73 (14): 4508–14. https://doi.org/10.1128/AEM.00733-07.
Fiedler C. J., Schönher C., Proksch P., Kerschbaumer D. J., Mayr E., Zunabovic-Pichler M., Domig K. J., and Perfler R. (2018): Assessment of Microbial Community Dynamics in River Bank Filtrate Using High-Throughput Sequencing and Flow Cytometry. Frontiers in Microbiology 9. https://doi.org/10.3389/fmicb.2018.02887.
Gabrielli, Marco, Andrea Turolla, and Manuela Antonelli (2021): Bacterial Dynamics in Drinking Water Distribution Systems and Flow Cytometry Monitoring Scheme Optimization. Journal of Environmental Management 286 (May): 112151. https://doi.org/10.1016/j.jenvman.2021.112151
García, Francisca C., Ángel López-Urrutia, and Xosé Anxelu G. Morán. (2014): Automated Clustering of Heterotrophic Bacterioplankton in Flow Cytometry Data. Aquatic Microbial Ecology.
Gillespie S., Lipphaus P., Green J., Parsons S., Weir P., Juskowiak K., Jefferson B., Jarvis P., and Nocker A. (2014): Assessing Microbiological Water Quality in Drinking Water Distribution Systems with Disinfectant Residual Using Flow Cytometry. Water Research 65 (November): 224–34. https://doi.org/10.1016/j.watres.2014.07.029.
Griebler, C., and T. Lueders. (2009): Microbial Biodiversity in Groundwater Ecosystems. Freshwater Biology 54 (4): 649–77.
Hammes F., Berney M., Wang Y., Vital M., Köster O., and Egli T. (2008): Flow-Cytometric Total Bacterial Cell Counts as a Descriptive Microbiological Parameter for Drinking Water Treatment Processes. Water Research 42 (1–2): 269–77. https://doi.org/10.1016/j.watres.2007.07.009.
Hammes, Frederik, Michael Berney, and Thomas Egli. (2010): Cultivation-Independent Assessment of Bacterial Viability. In High Resolution Microbial Single Cell Analytics, 123–50. Advances in Biochemical Engineering / Biotechnology. Springer, Berlin, Heidelberg.
Hammes F., Broger T., Weilenmann H.-U., Vital M., Helbing J., Bosshart U., Huber P., Odermatt R. P., and Sonnleitner B. (2012): Development and Laboratory-Scale Testing of a Fully Automated Online Flow Cytometer for Drinking Water Analysis. Cytometry Part A 81A (6): 508–16. https://doi.org/10.1002/cyto.a.22048.
Hassard, Francis, and Rachel Whitton. (2019): Understanding the Use of Flow Cytometry for Monitoring of Drinking Water. DWI 70/2/321 Defra WT2215. Cranfield Water Science Institute.
Højris B., and Torben Skovhus (2018): Microbiological Sensors for the Drinking Water Industry.
Højris, Bo, Boesgaard Christensen S.-C., Albrechtsen H.-J., Smith C., and Dahlqvist M. (2016): A Novel, Optical, on-Line Bacteria Sensor for Monitoring Drinking Water Quality. Scientific Reports 6 (April): 23935. https://doi.org/10.1038/srep23935.
Huang, Xiao, Zheng Zhao, Dana Hernandez, and Sunny C. Jiang. (2016): Near Real-Time Flow Cytometry Monitoring of Bacterial and Viral Removal Efficiencies during Water Reclamation Processes. Water 8 (10): 464.
Koch, Christin, Susanne Günther, Adey F. Desta, Thomas Hübschmann, and Susann Müller. (2013): Cytometric Fingerprinting for Analyzing Microbial Intracommunity Structure Variation and Identifying Subcommunity Function. Nature Protocols 8 (1): 190–202.
Lackner, Maximilian, Philipp Stadler, and Wilhelm Grabow (2017): Handbook of Online and Near-Real-Time Methods in Microbiology.
Lebaron, Philippe, Pierre Servais, Helene Agogué, Claude Courties, and Fabien Joux. (2001): Does the High Nucleic Acid Content of Individual Bacterial Cells Allow Us To Discriminate between Active Cells and Inactive Cells in Aquatic Systems? Applied and Environmental Microbiology 67 (4): 1775–82.
Liu, Gang, Ya Zhang, Ed van der Mark, Aleksandra Magic-Knezev, Ameet Pinto, Bartholomeus van den Bogert, Wentso Liu, Walter van der Meer, and Gertjan Medema. (2018): Assessing the Origin of Bacteria in Tap Water and Distribution System in an Unchlorinated Drinking Water System by SourceTracker Using Microbial Community Fingerprints. Water Research 138 (July): 86–96.
Kötzsch, Stefan, and Michael Sinreich. (2014): Zellzahlen Zum Grundwasser – Bestimmung Mittels Durchflusszytometrie. Aqua & Gas.
McEvoy B., Lynch M., and Rowan N. J. (2021): Opportunities for the Application of Real-Time Bacterial Cell Analysis Using Flow Cytometry for the Advancement of Sterilization Microbiology. Journal of Applied Microbiology 130 (6): 1794–1812. https://doi.org/10.1111/jam.14876.
Prest, E. I., F. Hammes, S. Kötzsch, M. C. M. van Loosdrecht, and J. S. Vrouwenvelder. (2013): “Monitoring Microbiological Changes in Drinking Water Systems Using a Fast and Reproducible Flow Cytometric Method.” Water Research, Microbial ecology of drinking water and wastewater treatment, 47 (19): 7131–42.
Van Nevel, S., S. Koetzsch, H.-U. Weilenmann, N. Boon, and F. Hammes. (2013): Routine Bacterial Analysis with Automated Flow Cytometry. Journal of Microbiological Methods 94 (2): 73–76.
Van Nevel S., Koetzsch S., Proctor C. R., Besmer M. D., Prest E. I., Vrouwenvelder J. S., Knezev A., Boon N., and Hammes F. (2017): Flow Cytometric Bacterial Cell Counts Challenge Conventional Heterotrophic Plate Counts for Routine Microbiological Drinking Water Monitoring. Water Research 113 (April): 191–206. https://doi.org/10.1016/j.watres.2017.01.065.
Proctor, Caitlin R., Michael D. Besmer, Timon Langenegger, Karin Beck, Jean-Claude Walser, Martin Ackermann, Helmut Bürgmann, and Frederik Hammes. (2018): Phylogenetic Clustering of Small Low Nucleic Acid-Content Bacteria across Diverse Freshwater Ecosystems. The ISME Journal, February, 1.
Rinke, Christian, Patrick Schwientek, Alexander Sczyrba, Natalia N. Ivanova, Iain J. Anderson, Jan-Fang Cheng, Aaron Darling, et al. (2013): Insights into the Phylogeny and Coding Potential of Microbial Dark Matter. Nature 499 (7459): 431–37.
Rogers, Wade T., Allan R. Moser, Herbert A. Holyst, Andrew Bantly, Emile R. Mohler, George Scangas, and Jonni S. Moore. (2008): Cytometric Fingerprinting: Quantitative Characterization of Multivariate Distributions. Cytometry Part A 73A (5): 430–41.
Sadler M. C., Senouillet J., Kuenzi S., Grasso L., and Watson D. C. (2020): Computational Surveillance of Microbial Water Quality With Online Flow Cytometry. Frontiers in Water 2. https://doi.org/10.3389/frwa.2020.586969.
Safford, Hannah R., and Heather N. Bischel. (2019): Flow Cytometry Applications in Water Treatment, Distribution, and Reuse: A Review. Water Research 151 (March): 110–33.
Schleich C., Chan S., Pullerits K., Besmer M. D., Paul C. J., Rådström P., and Keucken A. (2019): Mapping Dynamics of Bacterial Communities in a Full-Scale Drinking Water Distribution System Using Flow Cytometry. Water 11 (10): 2137. https://doi.org/10.3390/w11102137.
SLMB (2012): Methodenschrift 333.1: Bestimmung Der Totalzellzahl Und Des Quantitativen Verhältnisses Der Zellen Niedrigen Bzw. Hohen Nukleinsäuregehaltes in Süsswasser Mittels Durchflusszytometrie. In Schweizerisches Lebensmittelbuch. Schweizer Bundesamt für Gesundheit, Bern.
Stocks, S. M. (2004): Mechanism and Use of the Commercially Available Viability Stain, BacLight. Cytometry Part A 61A (2): 189–95.
Tian, Renmao, Daliang Ning, Zhili He, Ping Zhang, Sarah J. Spencer, Shuhong Gao, Weiling Shi, et al. (2020): Small and Mighty: Adaptation of Superphylum Patescibacteria to Groundwater Environment Drives Their Genome Simplicity. Microbiome 8 (1): 51.
Vera G. A. de, and Wert, E. C. (2019): Using Discrete and Online ATP Measurements to Evaluate Regrowth Potential Following Ozonation and (Non)Biological Drinking Water Treatment. Water Research 154 (May): 377–86. https://doi.org/10.1016/j.watres.2019.02.006.
Vergine P., Amalfitano S., Salerno C., Berardi G., and Pollice A. (2020): Reuse of Ultrafiltered Effluents for Crop Irrigation: On-Site Flow Cytometry Unveiled Microbial Removal Patterns across a Full-Scale Tertiary Treatment. Science of The Total Environment 718 (May): 137298. https://doi.org/10.1016/j.scitotenv.2020.137298.
Wang, Yingying, Frederik Hammes, Nico Boon, Mohamed Chami, and Thomas Egli. (2009): Isolation and Characterization of Low Nucleic Acid (LNA)-Content Bacteria. The ISME Journal 3 (8): 889–902.
Wang, Yingying, Frederik Hammes, Karen De Roy, Willy Verstraete, and Nico Boon. (2010): Past, Present and Future Applications of Flow Cytometry in Aquatic Microbiology. Trends in Biotechnology 28 (8): 416–24.
Wielen, Paul van der, and Dick van der Kooij. (2013): Microbial Growth in Drinking-Water Supplies. Problems, Causes, Control and Research Needs. IWA Publishing
Zunabovic-Pichler M., Mayr E., Schönher C., Fiedler C., Kerschbaumer D., Proksch P., and Perfler R. (2018): Durchflusszytometrie in der Wasserversorgung. Mikrobiologische Charakterisierung von Einflussfaktoren Und Zustandsveränderungen Bei Der Wasserversorgung. Wien: Bundesministerium für Nachhaltigkeit und Tourismus.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schönher, C., Proksch, P., Kerschbaumer, D. et al. “Every cell counts”—experiences with flow cytometry for Austrian drinking water supply. Österr Wasser- und Abfallw 73, 501–511 (2021). https://doi.org/10.1007/s00506-021-00802-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00506-021-00802-z