Abstract
Atomically-thin two-dimensional (2D) materials like graphene have been suggested as ultimately thin corrosion barriers and functional coatings for modern metallurgical alloys. The challenges of chemical vapour deposition (CVD) of such 2D materials, particularly graphene, on modern metallurgical alloys are discussed and reviewed here, focusing on the key problems with the metallurgical alloys’ often limited catalytic activity towards 2D materials growth and the key need to preserve the metallurgical alloys’ bulk properties during the high temperature 2D materials CVD processes. Using graphene CVD on NiTi (Nitinol) shape memory alloys as a case study, we illustrate the constraints arising from low catalytic activity and tendency to form oxides due the Ti in the NiTi alloy in terms of graphene growth results. We show that, by using a scalable low-temperature CVD process at 650 to 750 °C, we can deposit fully covering carbon films on the NiTi, albeit at limited structural quality. Notably, we also demonstrate that our CVD process does not degrade the bulk microstructure of the NiTi during carbon deposition and, importantly, leaves the crystallographic shape memory effect evolution intact. This underscores the potential of CVD for depositing graphene films on NiTi alloys while emphasizing the necessity for further exploration of CVD conditions to achieve high-quality graphene deposits akin to those on prior widely investigated dedicated (often sacrificial) high-purity metal substrates such as Ni.
Zusammenfassung
Atomar dünne zweidimensionale (2D) Materialien wie Graphen wurden als ultimativ dünne Korrosionsbarrieren und funktionelle Beschichtungen für moderne metallurgische Legierungen vorgeschlagen. Die Herausforderungen der chemischen Gasphasenabscheidung (chemical vapour deposition, CVD) solcher 2D-Materialien, insbesondere Graphen, auf modernen metallurgischen Legierungen werden hier diskutiert und überprüft, wobei der Schwerpunkt auf den Hauptproblemen liegt bezüglich der begrenzten katalytischen Aktivität der metallurgischen Legierungen für das Wachstum von 2D-Materialien und der Notwendigkeit, die Mikrostruktur und „bulk“-Eigenschaften der metallurgischen Legierungen während der Hochtemperatur-2D-Materialien-CVD-Prozesse zu erhalten. Anhand der Graphen-CVD auf Formgedächtnislegierungen aus NiTi (Nitinol) als Fallstudie veranschaulichen wir die Einschränkungen, die sich aus der geringen katalytischen Aktivität und der Tendenz zur Bildung von Oxiden aufgrund des Ti in der NiTi-Legierung im Hinblick auf die Ergebnisse des Graphenwachstums ergeben. Wir zeigen, dass wir mit einem skalierbaren Niedertemperatur-CVD-Prozess bei 650 bis 750 °C voll bedeckende Kohlenstofffilme auf dem NiTi abscheiden können, wenn auch mit begrenzter struktureller Qualität. Insbesondere zeigen wir auch, dass unser CVD-Verfahren die Mikrostruktur des NiTi während der Kohlenstoffabscheidung nicht beeinträchtigt und vor allem den kristallographischen Formgedächtniseffekt intakt lässt. Dies unterstreicht das Potenzial von CVD für die Abscheidung von Graphenfilmen auf NiTi-Legierungen und unterstreicht gleichzeitig die Notwendigkeit einer weiteren Erforschung der CVD-Bedingungen, um qualitativ hochwertigere Graphenabscheidungen zu erzielen, entsprechend denen auf zuvor umfassend untersuchten dedizierten hochreinen Metallsubstraten wie Ni.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Two-dimensional (2D) materials including graphene, the monolayered sp2 carbon material, are of key interest as ultimately thin functional coatings for modern metallurgical alloys [1,2,3,4,5]. This is because graphene offers virtual impermeability to gases, liquids, and ionic species, suggesting it as an ultimately thin corrosion barrier material [1,2,3,4,5]. Graphene is also chemically inert, temperature stable and has good biocompatibility [1,2,3,4,5,6,7,8,9,10]. Additionally, when its properties are normalized to its atomic thinness, graphene is an exceptional electrical conductor, has high thermally conductivity, and exhibits extraordinary mechanical properties [1,2,3,4,5]. It is the strongest crystal reported to date with still significant mechanical flexibility [2, 3] and, under some conditions, superlubricity [11, 12], suggesting also sufficient durability in metallurgical (low wear) applications.
Despite this, the actual realization of graphene as an ultrathin functional coating on metallurgical alloys has so far turned out to be challenging [4, 5, 13,14,15,16,17,18,19,20,21,22]. The dichotomy between reported record graphene properties and its, by comparison, lower performance in metallurgical testing results from the to date still insufficient control over scalable graphene growth in metallurgical application scenarios.
Growth of 2D materials incl. graphene on metallurgical alloys remains an unsolved challenge [4, 23]. It requires good interfacing and control over 2D materials’ defect levels, chemical compositions, layer numbers, lateral crystal sizes, homogeneity, and coverage. Homogeneous coverage over complex three-dimensional shapes, heterogeneous alloy microstructures, across polycrystalline substrate grains and over µm-surface roughness is essential, as is eventual industrial scalability. Importantly, alloy bulk microstructure and thus bulk functionality must not be degraded during 2D materials growth [4, 23, 24].
Two principal methods have been investigated for 2D coatings (incl. graphene) on alloys to date: The first method is a top-down wet-chemistry approach of sprayed or printed μm-thick film composed of atomically-thin 2D material flake building blocks from liquid phase exfoliation of a layered bulk material (i.e. graphite for graphene) [16,17,18,19,20,21,22]. This approach results not in atomically thin films overall, but rather μm-thick films composed of atomically thin building blocks. This can also often result in only partially covering, inhomogeneous 2D aggregates with non-ideal substrate interfacing and uncontrolled layer numbers [16,17,18,19,20,21,22].
The second method is the chemical vapour deposition (CVD) of 2D materials, which is a bottom-up approach in which gaseous precursors are flown at elevated temperatures over the desired growth support (here the metal alloys), leading to precursor breakdown and then 2D materials growth. For metallurgical applications, the coating of alloys with 2D materials incl. graphene by CVD is arguably the most scalable and applicable route for real-world use. This is because CVD is industrially scalable, can cover complex three-dimensional shaped substrates up to large sizes, and offers potentially excellent interfacing of defect-free, homogeneous 2D films on the alloy specimens. Notably, however, in 2D materials CVD, the growth surface has an active catalytic role [25,26,27,28,29,30,31,32]. This is due to the atomic thinness of the deposited 2D material and unlike the comparatively inert role that the substrate plays in CVD of traditional μm thick films [24]. Because of this active role of the substrate, close matching of 2D materials CVD conditions (temperature profiles, precursor fluxes etc.) with a metal alloys’ complex and heterogeneous surface and diffusional bulk properties is required, which to date has not been achieved even for the most basic metallurgical materials such as simple steels.
CVD has enabled the growth of high quality graphene and other 2D materials films (e.g. 2D hexagonal boron nitride) but so far only on dedicated high-purity metal growth catalyst supports with simple microstructures (e.g., Cu, Ni, partly Fe; advances to which also our work contributed to [25,26,27,28,29, 31]). Their microstructures do not have to be retained during growth as subsequently 2D materials are often removed from the catalysts and transferred to other device stacks. A simple translation of the progress on dedicated high-purity catalysts to modern metallurgical alloys is, however, difficult for two key reasons.
The first reason is the required active role of the growth substrate in 2D materials CVD via surface catalytic activity [25,26,27,28,29,30,31,32]. A close matching of substrate properties and 2D CVD conditions (temperature profiles, precursor fluxes, pre-treatments etc.) is required for each substrate individually [33, 34]. Already for the dedicated high-purity catalysts, this matching has been a formidable (but now well addressed) challenge [35]. For the much more complex heterogeneous microstructures of modern metallurgical alloys, such matching remains much more elusive. Even minor changes in composition and microstructure can drastically change (and often deteriorate) 2D material growth results. Generally, CVD of graphene on catalytically active metals, such as Ni, Co, Fe, and Cu, is much more readily achievable but already becomes more difficult when additional alloying elements are added to the substrate (which is the case in practically all modern metallurgical alloys). Furthermore, CVD of graphene on strong carbide formers, such as refractory Ti or Ta, is much more challenging as growth of a carbide from the carbon influx from the gaseous hydrocarbon graphene precursor into the substrate bulk at the elevated temperature can be preferred over nucleation of the graphene [15, 36]. Refractory substrates are also difficult to coat with graphene because the substrates readily form oxides with the residual oxygen and water in scalable CVD systems, whereby oxide formation often further impedes graphene CVD that typically requires a reduced metallic state to be catalytically active for growth [37]. For these reasons, individual (re-)calibration of 2D materials CVD conditions may be necessary for every different alloy composition.
The second reason is that the microstructure of the underlying metallurgical alloy must be retained during the 2D materials CVD process. Otherwise successfully coating a metallurgical alloy with a 2D material such as graphene may lead to overall functional degradation of the 2D material/support stack if the 2D material is grown well but concurrently the underlying metallurgical substrate properties (e.g. mechanical properties) have drastically degraded by undesired changes to substrate alloy microstructure during the high temperature 2D materials CVD process due to the alloy’s elements diffusion at the elevated temperatures and/or in-diffusion of 2D materials constituent elements (for graphene: carbon) into the alloy. This second aspect of the requirement to retain metallurgical substrate microstructure has to date received little attention in the 2D materials work.
In this contribution we highlight both these current challenges of (i.) often lacking active role of the growth substrate for graphene CVD and (ii.) the requirement of retention of metallurgical substrate bulk microstructure by studying the challenges of graphene CVD on NiTi shape memory alloys.
NiTi shape memory alloys (Nickel-Titanium, also called Nitinol) are roughly equimolar alloys consisting of nickel (Ni) and titanium (Ti) with extraordinary properties incl. a shape memory effect (SME) and superelasticity [38, 39]. The SME consists of a phase transition between a low temperature martensitic phase and higher temperature austenitic phase in NiTi. To use the SME, the NiTi can be shaped during the higher temperature austenite phase whereby this shape is then “memorized”. At the lower temperature in the martensite phase, the NiTi can then be deformed. After this deformation, the NiTi can still regain its previously established “memorized” shape at a later point by the introduction of heat and thus a reversion back to the austenitic phase. Thereby this SME makes the deformation in the martensitic phase reversible via cycling between martensitic and austenitic NiTi phases. The transition temperature between martensitic and austenitic phase can be engineered by exact Ni:Ti composition and material treatments and is typically controllable in the range from room temperature to ~100 °C [40].
The SME and the related superelasticity make NiTi an intriguing choice for various uses including in, e.g. the medicinal field as kidney stone extraction nets or cardiovascular stents [38]. In the context of such medical applications, Ni is, however, toxic for the human body and therefore direct contact with the NiTi alloy should be avoided. Medical NiTi implants can otherwise suffer from a lack of cell adhesion, proliferation, thrombosis, and metal leaching (incl. toxic Ni) after a sustained contact with flowing blood inside the body [7]. This can lead to strong immune responses that can interfere with the device functionality and even damage the device. It is therefore necessary to control and limit the interaction of the NiTi device with the surrounding biological environment and improve biocompatibility of the NiTi device. This can be achieved by coating the NiTi with a protective layer that should ideally be chemically inert, smooth, have a high adhesion strength, and be biocompatible [38].
As outlined above, graphene combines biocompatibility with chemical inertness and barrier capability and reasonable mechanical properties. For these reasons graphene coatings on NiTi substrates have been investigated in the past. The so far studied approaches include wet-chemistry deposited films consisting of exfoliated graphene building blocks [6], graphene CVD-grown on sacrificial high-purity Cu substrates with a subsequent mechanically transfer to NiTi [7, 8] and first attempts of direct graphene CVD on NiTi [9, 10].
For instance, one study employed graphene CVD grown on sacrificial (well calibrated) Cu supports with the graphene film subsequently released from the Cu and transferred to a NiTi shape memory alloy target substrate [7, 8]. Even this simply transferred graphene with non-ideal 2D material/substrate interaction has been shown to provide good corrosion protection and inhibit toxic Ni2+-ion release from the NiTi supports [7, 8]. For a better substrate interaction and adhesion, the direct growth (and not only transfer) of graphene on NiTi would be preferred, however. Towards this goal, another prior work reported so called “wormlike” graphene films that have been deposited by CVD directly on NiTi at 1050 °C, with variations in layer number and overall homogeneity [9]. Nevertheless this “wormlike” graphene coating has also been shown to already enhance biological activity compared to the bare NiTi substrate. This research on the “worm-like” graphene from Li et al. [9] also investigated the growth mechanism on NiTi under their conditions and showed that, after their CVD process, the surface is Ni deprived and a TiC layer has formed during the successful “wormlike” graphene growth. Li et al. also reported a native oxide layer on the substrate and the fact that rutile TiO2 was persistent under their conditions up to 950 °C. At temperatures above 1000 °C, no oxide was detected and instead a prominent TiC phase was measured, which they identify as the active phase for graphene growth. These prior findings highlight that changes to the metallurgical substrate (incl. the NiTi here) are commonplace at the elevated temperature graphene CVD conditions, as generally eluded to above.
2 Methods
Here we survey low-temperature (650 to 750 °C) CVD conditions for graphene growth on NiTi using acetylene (C2H2) as hydrocarbon precursor. The conditions are based on prior work to grow graphene on Ni [26, 27]. We have chosen these rather lower CVD temperatures to reduce the propensity of the NiTi substrates to undergo drastic bulk microstructure changes/degradation during the CVD process. C2H2 is chosen because it is the ideal hydrocarbon precursor for such lower temperatures due to its high reactivity down to ~450 °C for graphene growth [26]. We employ a custom-built hot-wall CVD system consisting of a quartz-tube (GVB, EN08NB) in a split-tube furnace (Carbolite Gero Split tube furnace—HZS 12/600), which is pumped by a rotary vane pump (Vacuubrand RZ 2.5) and small turbo molecular pump (VARIAN, Turbo‑V 70LP) combination to a base pressure of ~5 × 10−3 mbar. Process gases H2 (Messer 2.1, 99.1% purity) and C2H2 (Messer 2.6, 99.6% purity) are fed via a manual flow-controller and an electronic mass flow controller (Bronkhorst EL-FLOW select), respectively. We test several combinations of CVD conditions regarding pre-treatment in vacuum or H2 gas, presence of H2 during hydrocarbon exposure, temperatures (650 to 750 °C), and C2H2 flux. We first heat the samples either in base pressure vacuum or a reducing H2 atmosphere (~1 mbar) to their growth temperature, leave them at that temperature for pre-treatment in either base vacuum or H2 ~1 mbar atmosphere for 30 min, and then introduce the C2H2 flow either over vacuum or on top of the H2 for growth for 30 min. After 30 min of growth, the C2H2 and the heater are switched off and the samples are left to cool naturally (~30 °C/min) to room temperature. We survey C2H2 fluxes of 0.1 to 1 sccm and temperatures from 650 to 750 °C. We note that, with respect to growth on NiTi, the growth results from all surveyed CVD conditions are similar. As substrates we investigate commercial NiTi foils of 127 µm thickness (Alfa Aesar, Nitinol foil, 45514, flat annealed) with a stated transition temperature martensite to austenite of ~45 °C. An optical micrograph and a Raman spectrum of the as received NiTi foils are shown in Fig. 1. The optical microscopy and Raman confirm the as received NiTi foils to be of homogeneous appearance without signs of surface oxides or prior carbon coverage. In the same CVD runs, we also add elemental foil samples of the NiTi constituent elements, Ni (Alfa Aesar Puratonic 99.994% Nickel foil, 12046, 100 µm thickness), and Ti (Alfa Aesar, 99.99% Titanium foil, 13976, 127 µm thickness) to explore differences in carbon growth behaviour for the individual elements against their equimolar alloy. It should be noted that for Ni we expect reasonable graphitic film growth under these conditions based on our prior work [26, 27], while refractory Ti is expected to behave as a hard to grow on substrate under these temperatures based on prior work by others on similar refractory Ta substrates [15, 36].
We characterize our samples by using Raman spectroscopy (WITec alpha 300 RSA+) with excitation at 532 nm and a combined optical microscope. Temperature-programmed in situ X‑ray diffractometry (XRD) is conducted in a PANalytical X’Pert Pro diffractometer in Bragg-Brentano geometry using Cu Kα1,2 radiation filtered with an BBHD mirror and an X’Celerator linear detector. For in situ monitoring of experiments, an Anton Paar HTK 1200 N high temperatur chamber was used to cycle the NiTi samples between room temperature and 100 °C in vacuum of ~10−2 mbar to check for crystallographic SME evolution. The sample temperature is controlled directly via a thermocouple and direct environmental heating. The diffractograms were evaluated using the PANalytical program suite HighScorePlus. A background correction was performed. The phase assignment is based on the International Center for Diffraction Data (ICDD) PDF4+ database.
3 Results
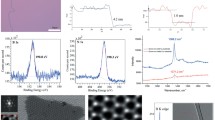
Figure 2 shows optical microscopy images and point localized Raman spectra of the three sample surfaces (NiTi, Ti, Ni) after a typical CVD run with 1 mbar H2 and 1 sccm C2H2 at ~750 °C. The samples exhibit visually distinct surfaces, already indicating differences in carbon growth.
Optical microscopy images of NiTi, Ti, and Ni (labelled) surfaces after CVD in 1 mbar H2 and 1 sccm C2H2 at 750 °C. Point-localized Raman spectra of the sample surfaces (colour-coded in the optical micrographs and Raman spectra; for Ti a typical spectrum is shown) showing surface oxidation and amorphous carbon signals for NiTi and Ti substrates and graphene coating for the Ni substrate. Peaks indicating TiO2 are indicated [44, 45] as are the carbon’s/graphene’s D, G and 2D peaks [41]
On the reference Ni, a carbon coating of mixed multilayered and monolayered graphene with very low defect levels was produced as evidenced by the sharp G and 2D peaks in the Raman spectra and the lack of an appreciable defect-related D peak [31, 41]. We note, however, that 1 sccm C2H2 is a too high flux of carbon feeding for Ni compared to our prior optimized homogeneous monolayered Ni recipes [26, 27], which translates to a too fast carbon feeding on the highly catalytically active Ni substrates leading to the observed mixed multilayer and monolayer graphene growth [31]. Ni would thus show better controlled graphene growth at lower carbon fluxes (< 0.1 sccm C2H2 in our system) [26, 27]. We, however, note that, for the NiTi and Ti substrates, we find that C2H2 fluxes < 0.1 sccm under otherwise constant conditions led to diminishing carbon coverage down to practically no carbon-related Raman signals after CVD (not shown). Therefore only the 0.1 to 1 sccm C2H2 results are presented here, and the Ni results serve only as an internal reference in terms of certainly achievable graphitization under these conditions with an established graphene CVD catalyst. We note that the Ni shows no signs of surface oxidation after CVD growth in Raman. This shows that our CVD system employed here has suitable low oxygen and water residual trace levels for typical graphene catalysts [42, 43].
In contrast to the clear graphitic Raman features and high structural graphene quality on Ni, the NiTi shows carbon deposition of much lower structural quality, whereby the carbon Raman spectra on NiTi in Fig. 2 are best attributed to an amorphous carbon film fully covering the substrate. This is evidenced by the broad merging D and G peaks and the lack of an appreciable 2D peak [41]. Additionally, the NiTi shows clear signs of Ti-oxide (TiO2) formation on the surface during the CVD process in the Raman in Fig. 2 [44, 45]. This implies that the scalable CVD conditions that we have applied are low enough in residual oxygen and water traces in the process atmosphere to avoid oxidation of Ni but that, for the addition of refractory Ti, which is an much stronger oxygen getter than Ni, our vacuum conditions are not sufficiently controlled with respect to oxygen/water traces to avoid oxidation during CVD for the NiTi sample. The formation of the amorphous carbon layer is found to be present all over on the NiTi surface in Fig. 2, whereby the carbon thickness appears to vary across the field of view, implying growth of a microstructure comprised of amorphous carbon regions of varying thickness. The formation of the Ti-oxide is also indicated by the change in colour in the optical micrographs in Fig. 2 after CVD compared to the as received state of the NiTi foil in Fig. 1.
We note that in terms of Raman quality our CVD results on the NiTi in Fig. 2 are not too dissimilar to prior literature on graphene CVD on NiTi and were in particular grown at much lower temperature (650–750 °C instead of ~1000 °C) [7, 8]. Compared to established recipes on other (non-refractory) substrates, the structural quality of the deposited carbon film (“amorphous carbon”) is, however, still quite poor and open to improvement. Additionally, concurrently to the amorphous carbon deposition, surface oxidation of the NiTi substrate has occurred, which is also undesired.
The Raman spectrum for the Ti reference in Fig. 2 similarly shows only growth of an amorphous carbon film and a similar surface oxidation to TiO2 under our CVD conditions. A comparison of the reference growths on Ni and Ti indicates that the low structural quality growth results on NiTi arise from the Ti in the NiTi compound. This implies that in the 50:50 mixture of Ni and Ti in the NiTi is, in terms of catalytic properties toward carbon deposition and resistance against oxidation, more dominated by the Ti properties than by the Ni properties. This highlights the problem of lacking activity for graphene CVD from an alloy substrate (here NiTi) even if one principal component element itself is usually catalytically highly active (here Ni) [26, 27].
To further improve our graphene CVD on NiTi, future work will therefore need to explore a wider set of kinetic conditions (temperatures, pre-treatments, carbon precursors and feeding rates etc.) to overcome the limitation of insufficient substrate catalytic activity towards graphene growth from the Ti component.
Nevertheless, our carbon films on NiTi grown at 650–750 °C presented here are not too dissimilar in structural quality to prior work and grown at substantially lower temperatures [7, 8]. Therefore, we proceed to check if the CVD process has detrimentally impacted on the NiTi bulk microstructure properties [10], which would be a highly detrimental factor in 2D materials CVD on metallurgical substrates, as outlined above, or if the NiTi bulk microstructure functionality has been retained throughout the graphene CVD process. A key test for the preservation of bulk microstructure functionality in NiTi is the exhibition of the SME, which is known to be dependent on the preservation of a suitable martensitic/austenitic NiTi microstructure evolution with temperature [46].
In order to therefore check if the basic SME property has been preserved post CVD, in situ XRD measurements were conducted on NiTi samples with a grown amorphous carbon layer after CVD. An example is shown in Fig. 3. A reference untreated NiTi sample and the CVD treated amorphous carbon coated NiTi sample were heated from room temperature in steps of 20 °C up to a temperature of 100 °C and then back to room temperature in two separate experiments. It should be noted that the expected martensite to austenite transition temperature for our NiTi substrates is ~45 °C.
a Temperature-resolved in situ XRD of as received NiTi substrate, showing a temperature cycle to 100 °C with majority martensitic (■, International Centre for Diffraction Data (ICDD), PDF-4+ database, powder diffraction file entry: 04-015-5901) and austenitic (▲, 04-002-7201) NiTi phases indicated. Additionally appeared minor peak phase(s) after 100 °C cycling remain currently unassigned. b Temperature-resolved in situ XRD of NiTi substrate after CVD process at 650 °C with vacuum pre-treatment and 0.1 sccm C2H2 and carbon growth similar to the results in Fig. 2. Majority austenitic NiTi (▲, 04-002-7201) and martensitic NiTi (■, 04-015-5901) and minority Ni3Ti (●, 04-007-1577) and minority TiO2 (◆, Rutile 04-001-7847, Anatase 04-004-3579) are indicated. We note that the background of the in situ XRD patterns of the CVD sample was manually treated in order to remove signals from the sample holder. This manual background treatment does not affect the measured structural evolution associated with the NiTi samples
The as received NiTi sample shows both martensitic and austenitic phases at room temperature, characteristic for NiTi of this composition [40]. As this untreated reference NiTi is heated to 40 °C, the martensitic peaks disappear and a complete change to the austenitic phase is observed. This is in excellent agreement with the stated martensite to austenite transition temperature of ~45 °C. No additional changes occur when increasing the temperature to 100 °C. Upon cooling, some of the martensitic features re-appear forming again a mixture of martensite and austenite as majority phases, although the austenitic peaks have become more prominent. This run is thereby typical for exhibiting the reversible martensitic/austenitic phase transformation (with some hysteresis) as a function of temperature that is responsible for the SME [46]. We therefore take this behaviour as a reference for a functional SME XRD evolution and compare in the following if the graphene CVD process has impacted on this SME XRD evolution.
For an amorphous carbon covered NiTi sample after graphene CVD, the XRD diffractogram at room temperature shows a similar mixture of majority martensitic and austenitic NiTi phases (International Centre for Diffraction Data (ICDD), PDF-4+ database, powder diffraction file entries: martensite: 04-015-5901, austenite: 04-002-7201) like in the as received reference NiTi sample. Additionally, however, minor rutile and anatase TiO2 signals as well as a minor Ni3Ti phase are detected (Rutile: 04-001-7847, Anatase: 04-004-3579, Ni3Ti: 04-007-1577). The TiO2 phases are readily explained by the surface oxidation already observed in Raman in Fig. 2. The existence of the Ni3Ti phase shows the extent of the surface oxidation, which formed TiO2 and left the NiTi subsurface region with an excess of Ni, leading to the formation of Ni3Ti. Upon heating to 40 °C, the CVD-treated NiTi sample undergoes the same majority transformation towards the full austenitic NiTi phase, analogous to the untreated as received NiTi reference sample. The oxide and Ni3Ti phases are stable and do not change. Again, no further changes occur when increasing the temperature to 100 °C. Upon cooling, the post CVD NiTi sample regains all of the majority martensitic phase peaks, converting back to the original state. Combined, the in situ XRD data shows that the majority martensite/austenite NiTi SME XRD evolution overall has not been affected by the CVD process. This is a strong indication that the CVD process has left the SME property of the NiTi intact, just as desired.
4 Conclusions
In summary, we have discussed and reviewed the challenges arising from the application of 2D materials CVD and, in particular, graphene CVD processes to metallurgical alloy substrates in terms of (i.) often lacking catalytic activity of the complex multi-elemental alloy microstructures for 2D materials growth and (ii.) the required retention of the bulk properties of the alloy during the high temperature CVD process. We exemplify these challenges by presenting graphene CVD results on the shape memory alloy NiTi. We show that, due to the low catalytic activity in the NiTi arising from the Ti, graphene growth is currently restricted to fully covering but low quality amorphous carbon coatings instead of high quality graphene. Also the propensity of Ti to oxide formation makes the NiTi samples susceptible to surface oxidation from oxygen/water residues in the CVD atmosphere, which is much less of a problem for standard graphene CVD catalysts like Ni. Nevertheless, our scalable low temperature recipe at 650 to 750 °C yields carbon films not too dissimilar to prior work on graphene CVD on NiTi. Notably, we also demonstrate here that our CVD process does not degrade the majority bulk martensite/austenite phases of the NiTi and, importantly, leaves their crystallographic SME evolution intact. This shows that CVD of 2D films on metallurgical NiTi substrates is possible while retaining their desired SME bulk functionality, but that more work on kinetic CVD condition exploration on the NiTi is necessary to achieve similarly high quality carbon deposits towards good quality graphene as we have prior achieved on dedicated high-purity elemental catalyst substrates like Ni.
References
Böhm, S.: Graphene against corrosion. Nature Nanotech 9, 741 (2014)
Novoselov, K.S., Fal, V., Colombo, L., Gellert, P., Schwab, M., Kim, K.: A roadmap for graphene. Nature 490, 192–200 (2012)
Ferrari, A.C., Bonaccorso, F., Fal’ko, V., Novoselov, K.S., Roche, S., Bøggild, P., Borini, S., Koppens, F.H.L., Palermo, V., Pugno, N., Garrido, J.A., Sordan, R., Bianco, A., Ballerini, L., Prato, M., Lidorikis, E., Kivioja, J., Marinelli, C., Ryhänen, T., Morpurgo, A., Coleman, J.N., Nicolosi, V., Colombo, L., Fert, A., Garcia-Hernandez, M., Bachtold, A., Schneider, G.F., Guinea, F., Dekker, C., Barbone, M., Sun, Z., Galiotis, C., Grigorenko, A.N., Konstantatos, G., Kis, A., Katsnelson, M., Vandersypen, L., Loiseau, A., Morandi, V., Neumaier, D., Treossi, E., Pellegrini, V., Polini, M., Tredicucci, A., Williams, G.M., Hong, H.B., Ahn, J.-H., Kim, M.J., Zirath, H., van Wees, B.J., van der Zant, H., Occhipinti, L., Di Matteo, A., Kinloch, I.A., Seyller, T., Quesnel, E., Feng, X., Teo, K., Rupesinghe, N., Hakonen, P., Neil, S.R.T., Tannock, Q., Löfwander, T., Kinaret, J.: Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 7, 4598–4810 (2015). https://doi.org/10.1039/C4NR01600A
Camilli, L., Yu, F., Cassidy, A., Hornekær, L., Bøggild, P.: Challenges for continuous graphene as a corrosion barrier. 2D Materials. 6, 022002 (2019)
Cui, G., Bi, Z., Zhang, R., Liu, J., Yu, X., Li, Z.: A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. , (2019)
Zhang, K., Zhang, H., Liu, P., Zhang, C., Li, W., Chen, X., Ma, F.: Electrophoretic deposition of graphene oxide on NiTi alloy for corrosion prevention. Vacuum 161, 276–282 (2019)
Podila, R., Moore, T., Alexis, F., Rao, A.M.: Graphene coatings for enhanced hemo-compatibility of nitinol stents. RSC Adv. 3, 1660–1665 (2013). https://doi.org/10.1039/c2ra23073a
Zhang, L., Duan, Y., Gao, Z., Ma, J., Liu, R., Liu, S., Tu, Z., Liu, Y., Bai, C., Cui, L., Yang, F., Liu, J., Kong, J., Li, Y.: Graphene enhanced anti-corrosion and biocompatibility of NiTi alloy. NanoImpact 7, 7–14 (2017). https://doi.org/10.1016/j.impact.2016.10.003
Li, J., Wang, G., Geng, H., Zhu, H., Zhang, M., Di, Z., Liu, X., Chu, P.K., Wang, X.: CVD growth of graphene on NiTi alloy for enhanced biological activity. ACS Applied Materials and Interfaces. 7, 19876–19881 (2015). https://doi.org/10.1021/acsami.5b06639
Zou, J., Wang, X., Zhang, P., Du, X.: Ultrafast flame coating of carbon and chemical vapor deposition of graphene on NiTi alloy to enhance its corrosion resistance. Diamond and Related Materials. 128, 109231 (2022)
Berman, D., Erdemir, A., Sumant, A.V.: Few layer graphene to reduce wear and friction on sliding steel surfaces. Carbon 54, 454–459 (2013)
Berman, D., Erdemir, A., Sumant, A.V.: Approaches for Achieving Superlubricity in Two-Dimensional Materials. Acs Nano 12, 2122–2137 (2018). https://doi.org/10.1021/acsnano.7b09046
Dumée, L.F., He, L., Wang, Z., Sheath, P., Xiong, J., Feng, C., Tan, M.Y., She, F., Duke, M., Gray, S.: Growth of nano-textured graphene coatings across highly porous stainless steel supports towards corrosion resistant coatings. Carbon 87, 395–408 (2015)
Zhu, M., Du, Z., Yin, Z., Zhou, W., Liu, Z., Tsang, S.H., Teo, E.H.T.: Low-temperature in situ growth of graphene on metallic substrates and its application in anticorrosion. ACS applied materials & interfaces. 8, 502–510 (2015)
Nazarova, M., Stora, T., Zhukov, A., Huang, K., Nair, R., Wang, Y., Yin, J., Holwill, M., Wang, Z., Mishchenko, A., Novoselov, K.S.: Growth of graphene on tantalum and its protective properties. Carbon 139, 29–34 (2018)
Chang, C.-H., Huang, T.-C., Peng, C.-W., Yeh, T.-C., Lu, H.-I., Hung, W.-I., Weng, C.-J., Yang, T.-I., Yeh, J.-M.: Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 50, 5044–5051 (2012)
Aneja, K.S., Bohm, S., Khanna, A., Bohm, H.M.: Graphene based anticorrosive coatings for Cr (VI) replacement. Nanoscale 7, 17879–17888 (2015)
Tong, Y., Bohm, S., Song, M.: The capability of graphene on improving the electrical conductivity and anti-corrosion properties of Polyurethane coatings. Appl Surf Sci 424, 72–81 (2017)
Krishnan, M.A., Aneja, K.S., Shaikh, A., Bohm, S., Sarkar, K., Bohm, H.M., Raja, V.: Graphene-based anticorrosive coatings for copper. Rsc Adv. 8, 499–507 (2018)
Raine, T.P., Istrate, O.M., King, B.E., Craster, B., Kinloch, I.A., Budd, P.M.: Graphene/Polyamide Laminates for Supercritical CO2 and H2S Barrier Applications: An Approach toward Permeation Shutdown. Advanced Materials. Interfaces 1800304(2018),
Yu, F., Camilli, L., Wang, T., Mackenzie, D.M., Curioni, M., Akid, R., Bøggild, P.: Complete long-term corrosion protection with chemical vapor deposited graphene. Carbon 132, 78–84 (2018)
Singhbabu, Y.N., Sivakumar, B., Choudhary, S.K., Das, S., Sahu, R.K.: Corrosion-protective reduced graphene oxide coated cold rolled steel prepared using industrial setup: A study of protocol feasibility for commercial production. Surf. Coatings Technol. 349, 119–132 (2018). https://doi.org/10.1016/j.surfcoat.2018.05.046
Halkjær, S., Iversen, J., Kyhl, L., Chevallier, J., Andreatta, F., Yu, F., Stoot, A., Camilli, L., Bøggild, P., Hornekær, L.: Low-temperature synthesis of a graphene-based, corrosion-inhibiting coating on an industrial grade alloy. Corros. Sci. 152, 1–9 (2019)
Dearnley, P.: Introduction to Surface Engineering. University Press, Cambridge (2017)
Kidambi, P.R., Bayer, B.C., Blume, R., Wang, Z.-J., Baehtz, C., Weatherup, R.S., Willinger, M.-G., Schloegl, R., Hofmann, S.: Observing Graphene Grow: Catalyst–Graphene Interactions during Scalable Graphene Growth on Polycrystalline Copper. Nano Lett., 13, 4769–4778 (2013)
Weatherup, R.S., Bayer, B.C., Blume, R., Ducati, C., Baehtz, C., Schlögl, R., Hofmann, S.: In Situ Characterization of Alloy Catalysts for Low-Temperature Graphene Growth. Nano Lett. 11, 4154–4160 (2011). https://doi.org/10.1021/nl202036y
Weatherup, R.S., Bayer, B.C., Blume, R., Baehtz, C., Kidambi, P.R., Fouquet, M., Wirth, C.T., Schlögl, R., Hofmann, S.: On the Mechanisms of Ni-Catalysed Graphene Chemical Vapour Deposition. ChemPhysChem 13, 2544–2549 (2012). https://doi.org/10.1002/cphc.201101020
Kidambi, P.R., Blume, R., Kling, J., Wagner, J.B., Baehtz, C., Weatherup, R.S., Schloegl, R., Bayer, B.C., Hofmann, S.: In situ observations during chemical vapor deposition of hexagonal boron nitride on polycrystalline copper. Chem. Mater. 26, 6380–6392 (2014)
Bayer, B.C., Caneva, S., Pennycook, T.J., Kotakoski, J., Mangler, C., Hofmann, S., Meyer, J.C.: Introducing Overlapping Grain Boundaries in Chemical Vapor Deposited Hexagonal Boron Nitride Monolayer Films. Acs Nano. 11, 4521–4527 (2017)
Caneva, S., Weatherup, R.S., Bayer, B.C., Blume, R., Cabrero-Vilatela, A., Braeuninger-Weimer, P., Martin, M.-B., Wang, R., Baehtz, C., Schloegl, R., Meyer, J.C., Hofmann, S.: Controlling Catalyst Bulk Reservoir Effects for Monolayer Hexagonal Boron Nitride CVD. Nano Lett. 16, 1250–1261 (2016). https://doi.org/10.1021/acs.nanolett.5b04586
Bayer, B.C., Bosworth, D.A., Michaelis, F.B., Blume, R., Habler, G., Abart, R., Weatherup, R.S., Kidambi, P.R., Baumberg, J.J., Knop-Gericke, A., Schloegl, R., Baehtz, C., Barber, Z.H., Meyer, J.C., Hofmann, S.: In Situ Observations of Phase Transitions in Metastable Nickel (Carbide)/Carbon Nanocomposites. J. Phys. Chem. C. 120, 22571–22584 (2016). https://doi.org/10.1021/acs.jpcc.6b01555
Caneva, S., Weatherup, R.S., Bayer, B.C., Brennan, B., Spencer, S.J., Mingard, K., Cabrero-Vilatela, A., Baehtz, C., Pollard, A.J., Hofmann, S.: Nucleation Control for Large, Single Crystalline Domains of Monolayer Hexagonal Boron Nitride via Si-Doped Fe Catalysts. Nano Lett. 15, 1867–1875 (2015)
Weatherup, R.S., Dlubak, B., Hofmann, S.: Kinetic Control of Catalytic CVD for High-Quality Graphene at Low Temperatures. Acs Nano. 6, 9996–10003 (2012)
Cabrero-Vilatela, A., Weatherup, R.S., Braeuninger-Weimer, P., Caneva, S., Hofmann, S.: Towards a general growth model for graphene CVD on transition metal catalysts. Nanoscale 8, 2149–2158 (2016)
Lin, L., Deng, B., Sun, J., Peng, H., Liu, Z.: Bridging the gap between reality and ideal in chemical vapor deposition growth of graphene. Chem. Rev. 118, 9281–9343 (2018)
Fan, X., Sun, J., Guo, W., Ke, X., Yan, C., Li, X., Dong, Y., Xiong, F., Fu, Y., Wang, L., Deng, J., Xu, C.: Chemical vapor deposition of graphene on refractory metals: The attempt of growth at much higher temperature. Synth. Met. 247, 233–239 (2019). https://doi.org/10.1016/j.synthmet.2018.12.016
Kidambi, P.R., Bayer, B.C., Weatherup, R.S., Ochs, R., Ducati, C., Szabó, D.V., Hofmann, S.: Hafnia nanoparticles—a model system for graphene growth on a dielectric. physica status solidi RRL Rapid Research Letters, 5, 341–343 (2011)
Duerig, T., Pelton, A., Stöckel, D.: An overview of nitinol medical applications. Mater. Sci. Eng. A 273, 149–160 (1999)
Bayer, B.C., Khan, A.F., Mehmood, M., Barber, Z.H.: Effect of substrate on processing of multi-gun sputter deposited, near-stoichiometric Ni2MnGa thin films. Thin Solid Films 518, 2659–2664 (2010). https://doi.org/10.1016/j.tsf.2009.08.030
Sanjabi, S., Sadrnezhaad, S.K., Yates, K.A., Barber, Z.H.: Growth and characterization of TixNi1−x shape memory thin films using simultaneous sputter deposition from separate elemental targets. Thin Solid Films 491, 190–196 (2005). https://doi.org/10.1016/j.tsf.2005.06.004
Ferrari, A.C.: Raman spectroscopy of graphene and graphite: disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun 143, 47–57 (2007)
Fuchs, D., Bayer, B.C., Gupta, T., Szabo, G.L., Wilhelm, R.A., Eder, D., Meyer, J.C., Steiner, S., Gollas, B.: Electrochemical Behavior of Graphene in a Deep Eutectic Solvent. ACS Appl. Mater. Interfaces 12, 40937–40948 (2020). https://doi.org/10.1021/acsami.0c11467
Fickl, B., Seifried, T.M., Rait, E., Genser, J., Wicht, T., Kotakoski, J., Rupprechter, G., Lugstein, A., Zhang, D., Dipolt, C., Grothe, H., Eder, D., Bayer, B.C.: Controllable Freezing Transparency for Water Ice on Scalable Graphene Films on Copper. arXiv preprint arXiv:2403.15629. (2024)
Ohsaka, T., Izumi, F., Fujiki, Y.: Raman spectrum of anatase, TiO2. J Raman Spectroscopy 7, 321–324 (1978). https://doi.org/10.1002/jrs.1250070606
Frank, O., Zukalova, M., Laskova, B., Kürti, J., Koltai, J., Kavan, L.: Raman spectra of titanium dioxide (anatase, rutile) with identified oxygen isotopes (16, 17, 18). Phys. Chem. Chem. Phys. 14, 14567–14572 (2012). https://doi.org/10.1039/c2cp42763j
Bayer, B.C., Sanjabi, S., Baehtz, C., Wirth, C.T., Esconjauregui, S., Weatherup, R.S., Barber, Z.H., Hofmann, S., Robertson, J.: Carbon nanotube forest growth on NiTi shape memory alloy thin films for thermal actuation. Thin Solid Films 519, 6126–6129 (2011). https://doi.org/10.1016/j.tsf.2011.03.044
Acknowledgements
B.C.B. and C.D. acknowledge funding to this work from the Austrian Research Promotion Agency (FFG) via the Production of the Future Programme of the Austrian Ministry of Climate Action, Environment, Energy, Mobility, Innovation and Technology (BMK) under project 879844-HARD2D. The authors acknowledge TU Wien Bibliothek for financial support through its Open Access Funding Programme.
Funding
Open access funding provided by TU Wien (TUW).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fickl, B., Heinzle, S., Gstöttenmayr, S. et al. Challenges in Chemical Vapour Deposition of Graphene on Metallurgical Alloys Exemplified for NiTi Shape Memory Alloys. Berg Huettenmaenn Monatsh 169, 357–365 (2024). https://doi.org/10.1007/s00501-024-01482-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00501-024-01482-4