Abstract
Microbiological methanation is planned in an underground natural gas reservoir. For this purpose, hydrogen is stored, which can lead to hydrogen embrittlement of steels. To simulate these field conditions, autoclave tests were performed to clarify the amount of absorbed hydrogen and to test whether this content leads to failure of the steels. Constant load tests and immersion tests with subsequent hydrogen analyses were performed. Tests under constant load have shown that no cracks occur due to hydrogen pressures up to 100 bar and temperatures at 25 °C and 80 °C. In these conditions, the carbon steels absorb a maximum of 0.54 ppm hydrogen, which is well below the embrittlement limit. Austenitic stainless steels absorb much more hydrogen, but these steels also have a higher resistance to hydrogen embrittlement. In H2S saturated solutions, the hydrogen uptake is ten times higher compared to hydrogen gas, which has caused fractures of several steels (high strength carbon steels, Super 13Cr, and Duplex stainless steel 2205).
Zusammenfassung
In unterirdischen Erdgaslagerstätten ist die mikrobiologische Methanisierung geplant. Dazu wird Wasserstoff eingelagert, was zu Wasserstoffversprödung von Stahl führen kann. Um diese Feldbedingungen nachzustellen, wurden Autoklavenversuche durchgeführt, um zu klären, wie viel Wasserstoff aufgenommen wird und ob dieser Gehalt zu einem Versagen der Stähle führt. Es wurden Versuche unter konstanter Last und Auslagerungsversuche mit anschließenden Wasserstoffanalysen durchgeführt. Versuche unter konstanter Last haben gezeigt, dass keinerlei Risse durch Wasserstoffdrücke bis 100 bar und Temperaturen bei 25 °C und 80 °C aufgetreten sind. Die Kohlenstoffstähle nehmen bei diesen Bedingungen maximal 0.54 ppm Wasserstoff auf, was deutlich unterhalb der Versprödungsgrenze liegt. Austenitische Stähle nehmen viel mehr Wasserstoff auf, allerdings weisen diese Stähle auch eine höhere Toleranz gegenüber Wasserstoff auf. In H2S gesättigten Elektrolyten ist die Wasserstoffaufnahme um den Faktor 10 höher als in Wasserstoffgas, was zu Brüchen mehrerer Stähle (hochfeste Kohlenstoffstähle, Super 13Cr und Duplex Stahl 2205) geführt hat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As the share of renewable energies increases, new challenges must also be faced. Periodically changing weather conditions lead to fluctuating power outputs, and the excess energy often has to be stored. The conversion of excess electricity into hydrogen by electrolysis is an option, but the lack of sufficient infrastructure for the storage and transportation of the gas is a problem. Unlike hydrogen, natural gas has a functioning storage and transport infrastructure. Methanation of hydrogen and carbon dioxide can be performed to obtain natural gas:
A new approach is to use methanogenic archaea that perform the methanation process [1]. The microorganisms can produce natural gas in an underground natural gas reservoir, where they occur naturally. In the presence of H2, the hydrogen embrittlement of steel components, such as casing and tubing, must be considered. This phenomenon, although already reported in 1874 by Johnson [2], is not yet fully understood.
With H2 and a corrosive environment involved, there are two main potential sources of absorbed hydrogen. The first one is the dissociation of H2 molecules:
The hydrogen molecule dissociates to two adsorbed hydrogen atoms Had. The atomic hydrogen can be absorbed by the material. Fig. 1 shows this process schematically.
Hydrogen absorption in a gaseous hydrogen environment [3]
According to Sieverts and Krumbhaar, the hydrogen solubility of metals increases with increasing temperatures [4]. This is depicted in Sieverts’s law:
where S0 is the solubility constant, p the partial pressure, ∆H the heat of solution, R the universal gas constant, and T the absolute temperature [5]. When CO2 dissolves in water, carbonic acid H2CO3 is formed [6]. The carbonic acid dissociates in two steps. The increasing concentration of H+ results in a lower pH, and the cathodic reaction is promoted [7]:
Simultaneously, anodic dissolution of iron takes place:
These two partial reactions are part of the following process [6]:
Further, the precipitation of siderite, FeCO3, can occur [6]:
This phenomenon is commonly known as sweet corrosion.
In acidic environments containing hydrogen sulfide, H2S, iron also dissolves according to Eq. 5, iron sulfide FeS is formed and H+ is produced [8]:
When H2S is present, it is commonly referred to as sour corrosion. In both sweet and sour corrosion, not all of the reduced H+ ions recombine to H2, as shown in Eq. 4. Some of them can get adsorbed (Had) and subsequently absorbed (Hab) [8]:
Thus, the second potential source of absorbed hydrogen is the corrosion reactions. It is also known that H2S hinders the recombination of hydrogen and, therefore, promotes the absorption of hydrogen [9, 10].
Whiteman and Troiano stated [11] that the amount of absorbed hydrogen necessary to produce hydrogen embrittlement is one or two orders of magnitude greater for austenitic stainless steels compared to steels with a bcc lattice. Although the ferrite with its bcc lattice enables fast diffusion of hydrogen atoms, it cannot dissolve so much of them compared to austenite with its densely packed fcc lattice [12]. The difference in diffusivity is four to five orders of magnitude, and that of the solubility is two to three orders of magnitude [13]. According to Tohme et al., there is a driving force to transport hydrogen from ferrite to austenite, which is referred to as up-hill diffusion [14]. Therefore, a duplex stainless steel, which is a mixture of both microstructures, is of interest for being tested in terms of hydrogen uptake and embrittlement.
2 Experimental Procedure
The resistance to hydrogen embrittlement and the hydrogen uptake of different material grades in various high-pressure media were investigated by means of autoclave tests.
Materials Investigated: to get a broad picture, tests were performed on a large variety of material grades. On the one hand, the carbon steels L80, P110 (both according to APIFootnote 1 5CT [15]), and 42CrMo4 (UNS G41400), on the other hand, the corrosion resistant alloys (CRAs) Super 13Cr, Duplex 2205, and Alloy 28 were investigated. Samples were taken from commercially available casing tube sections. The chemical composition of the investigated material grades is given in Table 1.
Table 2 gives the microstructure and grain size of the tested materials. For martensitic steel grades, the size of former austenite grains is listed as grain size.
The mechanical properties of the tested materials are listed in Table 3. Tensile tests were performed on small, non-standard tensile specimens with an initial gauge length of 25 mm and a diameter of 3 mm. The specimens were drawn at room temperature with a crosshead speed of 0.1 mm/min.
Autoclave tests in H2-containing media: the tests with hydrogen gas were conducted within autoclaves made of UNS N06625 (Alloy 625). Fig. 2 shows one of the used autoclaves.
Each autoclave contained three different specimens: an immersion specimen (Fig. 3a i) for measuring the hydrogen uptake, a coupon (Fig. 3a ii for determining the presence of pitting or other corrosion phenomena, and a small tensile specimen (Fig. 3a) iii ) for a constant load test (CLT). The load was applied to the CLT specimen with a spring made of a cobalt-base alloy and ceramic nuts (Fig. 3b), the latter ensuring electronic decoupling of the specimen from the more noble spring. The specimens were connected with PTFE parts and mounted in the autoclaves.
Afterwards, the vessels were evacuated and purged with argon several times to obtain very low partial pressures of oxygen and other atmospheric gases. Further, the autoclaves were filled with an aqueous test solution and various test gases (Fig. 4a and b). Finally, the autoclaves were mounted on rotating shafts in a heated chamber (Fig. 4c).
Tests were performed with two different partial pressures of hydrogen gas: 20 bar (290 psi) and 100 bar (1450 psi). In addition, the influence of 5 bar (73 psi) of CO2 gas was also investigated. In more than half of the tests, an aqueous NaCl solution (brine) with a chloride concentration of 15,000 mg/l was used. The tests were conducted at 25 °C (77 °F) as well as at 80 °C (176 °F, near-field conditions) and lasted for 30 days. Thus, each material was tested under ten different conditions.
The load for the CLTs was 90% of the specified minimum yield strength (SMYS). The load was applied by compressing a spring with a defined load and fixing it by connection to the respective specimen and two nuts. To simulate the regularly changing conditions in the gas well, the autoclaves were rotated with a speed of 1 RPM. Consequently, the specimens were periodically wetted with the aqueous electrolyte, if any.
Tests in H2S‑containing media: the tests with H2S were conducted in vessels normally used for laboratory testing of metals for resistance to sulfide stress cracking according to the NACE standard TM0177 [16]. The setup for these tests is shown in Fig. 5.
Immersion specimens were tested in glass cells with a volume of 6 liters (Fig. 5a). CLTs were conducted in sealed Method A vessels (Fig. 5b). CLT specimens were loaded as described earlier. The geometry of the specimens was the same as in the autoclave tests (Fig. 3a i, iii and b). This ensured the comparability of the results for the two different types of tests.
The medium for these tests was Solution A described in standard [16]. This is an acidified H2S‑saturated aqueous brine solution with 5.0 wt% NaCl and 0.5 wt% CH3COOH. For the immersion tests, 4 liters of solution per glass cell were used. The solution and the vessels were purged with inert gas prior to testing.
Evaluation Methods: Directly after the autoclave tests, the immersion specimens were removed from the vessels and immediately cooled in liquid nitrogen. Specimens immersed in the acidified H2S‑saturated aqueous brine solution were removed after 3 h, 30 h, and 336 h of loading and immediately cooled in liquid nitrogen, too. The cooled specimens were ground with silicon carbide paper (grit 120) to remove corrosion products, rinsed with acetone and blow-dried quickly prior to hydrogen analysis. The hydrogen content was measured with a thermal conductivity cell after hot extraction at 950 °C (1742 °F).
At the end of the autoclave tests and periodically during the H2S tests, the constant load specimens were checked for possible fractures.
3 Results
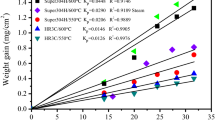
Constant Load Tests (CLTs): None of the specimens constantly loaded at 90% of the SMYS broke under the conditions tested in the autoclaves (20 bar H2, 100 bar H2, and 5 bar CO2). None of the unbroken specimens showed visible cracks under the stereo microscope. Fig. 6 shows the results of the constant load tests in acidified H2S‑saturated aqueous brine. The dashed line symbolizes the end of the test after 14 days.
The first specimens to fail in H2S were those made of P110. These were broken after only 10 min. They were followed by the 42CrMo4, failing after less than 80 min. The next material to fail was the Super 13Cr, after 195 min and less than 220 min. For both the L80 and Alloy 28, none of the specimens failed in H2S during the 336 h of testing. The same was observed for one of the Duplex 2205 specimens, with the second specimen failing after less than 48 h.
Hydrogen Content: The hydrogen contents of the carbon steels measured after 30 days of autoclave testing with various media at 25 °C and 80 °C (77 °F and 176 °F) are shown in Fig. 7.
In the uncharged condition, a hydrogen content of 0.10 ppm was measured for all three carbon steels. Autoclave testing without brine (dry gas) did not lead to significant amounts of absorbed hydrogen, except for tests with 100 bar H2 at 80 °C, where a maximum of 0.54 ppm was found for the 42CrMo4. The presence of brine in the autoclave generally led to a higher hydrogen content compared to the results for dry gas. Exceptions are the tests at 100 bar H2 and 80 °C, where the level of absorbed hydrogen does not differ significantly from tests without brine. The presence of CO2 led to an elevated hydrogen content at both temperatures tested, although the results are slightly lower than those measured after testing with hydrogen gas and the same electrolyte.
Fig. 8 shows the results for the CRAs tested under the same conditions. The CRAs charged in 20 and 100 bar of dry hydrogen gas at 25 °C did not show a hydrogen content significantly higher than that of the uncharged condition. A higher temperature (80 °C) increased the amount of hydrogen absorbed by Super 13Cr and Alloy 28, with higher pressure giving higher hydrogen content after dry gas tests. This behavior was found to be less pronounced for the Duplex 2205, since its hydrogen content was only slightly increased by a higher temperature and pressure of dry hydrogen gas.
As described for the carbon steels before, the presence of brine during autoclave testing with 20 and 100 bar H2 led to a higher hydrogen content compared to the results for dry gas. This effect was observed in the following combinations of materials and conditions: Super 13Cr at 25 °C (20 and 100 bar H2), Duplex 2205 at 25 °C (100 bar H2), and Duplex 2205 at 80 °C (20 and 100 bar H2), with the latter showing the most significant increase. In the remaining combinations, the addition of brine resulted in only a minor increase in the hydrogen content measured after testing, where for Alloy 28 the smallest differences were found.
CRAs tested in brine and 5 bar CO2 showed no significant change in hydrogen content. In Fig. 9 the hydrogen content of the materials tested in acidified H2S‑saturated aqueous brine solution with 5.0 wt% NaCl and 0.5 wt% CH3COOH (NACE Solution A) is shown.
For the carbon steels, after 3, 30, and 336 h of immersion in NACE Solution A, significant amounts of absorbed hydrogen were measured (Fig. 9b) compared to the uncharged condition (Fig. 9a). 42CrMo4 and P110 showed a maximum hydrogen content of 8.35 and 6.61 ppm, respectively, after 3 h of testing. For the L80, the highest result was 5.58 ppm measured after 30 h.
Only one of the CRAs showed a significant increase in the hydrogen content after immersion in the H2S‑saturated solution: In the Super 13Cr specimens, a maximum of 15.60 ppm was measured after 30 h of testing. For Duplex 2205 and Alloy 28, no substantial amount of hydrogen was absorbed.
4 Discussion
The hydrogen content of all carbon steels tested in hydrogen gas with a maximum pressure of 100 bar was low overall compared to the results obtained after immersion in the H2S‑saturated solution. For example, 42CrMo4 had a maximum hydrogen content of 0.54 ppm after being charged with 100 bar of dry H2 gas at 80 °C for 30 days, while testing in NACE Solution A for 3 h led to a hydrogen content of 8.35 ppm. Both the 42CrMo4 and the P110 loaded with 90% of the SMYS failed the sour gas test in NACE Solution A, but none of the specimens tested in H2 with up to 100 bar showed a substantial embrittlement.
The sour service grade L80 failed neither in H2 nor in H2S, although the latter led to a hydrogen content of up to 5.58 ppm. Since the L80 absorbed far less hydrogen when charged with 100 bar of H2 gas compared to immersion in the H2S saturated solution without failure, it seems to be suitable for application in an underground microbiological methanation facility with high pressure H2 gas.
The carbon steels and the Super 13Cr had a high hydrogen content in the beginning of the H2S test, with a decline towards the end of the test. For similar steels this behavior was already reported in the literature [17]. An explanation is the increasing formation of surface layers over time.
An increase of temperature and hydrogen gas pressure in the autoclaves led to a larger increase in the amount of hydrogen absorbed by the CRAs compared to the carbon steels tested under the same conditions.
The Super 13Cr failed after less than 4 h of testing in NACE Solution A. After 3 h of immersion in the H2S‑saturated solution, a hydrogen content of 6.97 ppm was found. Tests on this steel grade in 100 bar H2 at 80 °C with brine led to a hydrogen content of 6.17 ppm without failure. It seems that the application limit of Super 13Cr is somewhere around 7 ppm, which was not reached in tests with high pressure hydrogen gas.
The Duplex 2205 was the CRA containing the highest amount of absorbed hydrogen after 30 days of testing in 100 bar H2 at 80 °C with brine. It also showed a unique behavior in dry hydrogen gas at 80 °C: while in the Super 13Cr and the Alloy 28 a significantly increased hydrogen content was measured after testing under these conditions, the increase was much smaller for the duplex stainless steel. An explanation for this behavior could lie in the nature of its ferritic-austenitic microstructure.
In NACE Solution A, one of the specimens made of Duplex 2205 and loaded with 90% of the SMYS failed, even though the hydrogen uptake was not increased significantly. Since none of the CLT specimens made of the same material failed in the autoclave tests, although hydrogen contents of up to 14.21 ppm were measured, the reason for the failure in H2S should not be embrittlement by hydrogen.
Autoclave tests on CRAs with dry hydrogen gas at 25 °C showed that no appreciable hydrogen absorption takes place under these conditions. The addition of brine increases the hydrogen absorption of Super 13Cr and Duplex 2205. Alloy 28 did not show this behavior to the same extent.
The presence of CO2 increased the hydrogen content of the carbon steels tested in autoclaves under wet conditions. Since the specimens had a layer of dark grey corrosion products, the source of hydrogen was the corrosion reactions. The specimens made of CRAs showed no signs of corrosion, therefore no absorbed hydrogen was measured.
5 Conclusions
-
No cracks occurred under a constant load of 90% SMYS within 30 days of testing in rotated autoclaves containing up to 100 bar hydrogen gas with or without brine (15,000 mg/l chloride). The extent of hydrogen absorption of carbon steels was low, but still detectable.
-
The immersion of L80, 42CrMo4, and P110 in NACE Solution A (acidified H2S‑saturated aqueous brine solution) resulted in a much higher hydrogen content than autoclave tests with up to 100 bar H2. Among the carbon steels tested, only the L80 survived the H2S test under constant load of 90% SMYS. L80 seems to be suitable for application in an underground microbiological methanation facility with high pressure H2 gas.
-
It seems that the application limit of Super 13Cr is somewhere around 7 ppm of absorbed hydrogen, which was not reached in tests with 100 bar of hydrogen gas.
-
The Duplex 2205 was the CRA containing the highest amount of absorbed hydrogen after 30 days of autoclave testing in 100 bar H2 at 80 °C with brine. However, the hydrogen content of 14.21 ppm did not cause a substantial embrittlement.
-
Autoclave tests on CRAs with dry hydrogen gas at 25 °C showed that no appreciable hydrogen absorption takes place under these conditions. The addition of brine can increase the hydrogen uptake of certain alloys.
-
An increase of temperature and hydrogen gas pressure in the autoclaves led to a larger increase in the amount of hydrogen absorbed by the CRAs compared to the carbon steels tested under same conditions.
Notes
American Petroleum Institute (API), 1220 L St., N.W., Washington, DC 20005-4070
References
Burkhardt, M.; Busch, G.: Methanation of hydrogen and carbon dioxide, Applied Energy, 111 (2013), pp 74–79
Johnson, W. H.: On Some Remarkable Changes Produced in Iron and Steel by the Action of Hydrogen and Acids, Proceedings of the Royal Society of London, 23 (1875), pp 168–179
Louthan, M. R.: Hydrogen Embrittlement of Metals: A Primer for the Failure Analyst, Journal of Failure Analysis and Prevention, 8 (2008), no 3, pp 289–307
Sieverts, A.; Krumbhaar, W.: Über die Löslichkeit von Gasen in Metallen und Legierungen, Berichte der deutschen chemischen Gesellschaft, 43 (1910), pp 893–900
Rawls, G. B.; Adams, T.: Hydrogen production and containment, in: Somerday, B. P.; Gangloff, R. P. (Eds.): Gaseous hydrogen embrittlement of materials in energy technologies: Volume 1: The problem, its characterisation and effects on particular alloy classes, Cambridge: Woodhead Publishing Ltd, 2012
Dugstad, A.: Fundamental Aspects of CO2 Metal Loss Corrosion. Part I: Mechanism, Proceedings, NACE Corrosion Conference and Expo 2006, San Diego, California, 2006
Protopopoff, E.; Marcus, P.: Electrode Potentials, in: Cramer, S. D.; Covino, B. S. Jr. (Eds.): ASM Handbook Volume 13A: Corrosion: Fundamentals, Testing, and Protection, ASM International, Materials Park, OH, 10 (2003)
Boellinghaus, T.; Hoffmeister, H.; Klemme, J.; Alzer, H.: Hydrogen Permeation in a Low Carbon Martenistic Stainless Steel Exposed to H2S Containing Brines at Free Corrosion, NACE Corrosion 99, Houston, TX: NACE International, 1999
Kawashima, A.; Hashimoto, K.; Shimodaira, S.: Hydrogen Electrode Reaction and Hydrogen Embrittlement of Mild Steel in Hydrogen Sulfide Solutions, Corrosion, 32 (1976), no 8, pp 321–331
Iyer, R.N.; Takeuchi, I.; Zamanzadeh, Z.; Pickering, H.W.: Hydrogen Sulfide Effect on Hydrogen Entry into Iron—A Mechanistic Study, Corrosion, 46 (1990), no 6, pp 460–468
Whiteman, M. B.; Troiano, A. R.: Hydrogen Embrittlement Of Austenitic Stainless Steel, Corrosion, 21 (1965), pp 53–56
Wei, F.G.; Tsuzaki, K.: Hydrogen trapping phenomena in martensitic steels, in: Gaseous hydrogen embrittlement of materials in energy technologies: Volume 1: The problem, its characterisation and effects on particular alloy classes, eds. B. P. Somerday, R. P. Gangloff, Cambridge: Woodhead Publishing Ltd, 2012, pp 493–525
Olden, V.; Saai, A.; Jemblie, L.; Johnsen, R.: FE simulation of hydrogen diffusion in duplex stainless steel, International Journal of Hydrogen Energy, 39 (2014), pp 1156–1163
Tohme, E.; Barnier, V.; Christien, F.; Bosch, C.; Wolski, K.; Zamanzade, M.: SKPFM study of hydrogen in a two phase material. Experiments and modelling, International Journal of Hydrogen Energy, 44 (2019), pp 18597–18605
API 5CT 9th ed. (2011), Specification for Casing and Tubing, American Petroleum Institute, Washington, DC, 2011
TM0177–2016, Laboratory Testing of Metals for Resistance to Sulfide Stress Cracking and Stress Corrosion Cracking in H2S Environments, Houston, TX: NACE International, 2016
Holzer, C.: Crack growth and development of new high strength, sour gas resistant steels, PhD Thesis, Chair of General and Analytical Chemistry, Leoben: Montanuniversitaet Leoben, 2016
Acknowledgements
The authors would like to thank voestalpine BÖHLER Edelstahl GmbH & Co KG for providing material for manufacturing of autoclaves and voestalpine Tubulars GmbH & Co KG as well as Cécile Millet from Vallourec Research Center France for providing casing tube sections. Special thanks are due to the Austrian federal government’s Climate and Energy Fund for partly funding this research.
Funding
Open access funding provided by Montanuniversität Leoben.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trautmann, A., Mori, G. & Loder, B. Hydrogen Embrittlement of Steels in High Pressure H2 Gas and Acidified H2S-saturated Aqueous Brine Solution. Berg Huettenmaenn Monatsh 166, 450–457 (2021). https://doi.org/10.1007/s00501-021-01143-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00501-021-01143-w