Abstract
This work represents an extended abstract of a study presented at the PRES 2016 conference. Despite the fact that alternative processes such as Corex® and Finex® have already been established on industrial scale, the blast furnace route continues to be the most important process used to produce pig iron. Due to its importance, a variety of models of the blast furnace process have been developed in the past decades. In addition to this, a well-established analogue representation of blast furnace operation is given by the Rist operating diagram. The target of this work was to create a comprehensive blast furnace model in the process simulation platform of gPROMS ModelBuilder®, which enables the description of interdependencies between the main blast furnace process, raceway conditions as well as the overall thermodynamic process conditions within a single mathematical model. As an example of possible applications of the developed model, a detailed analysis of the model behaviour under varying coke substitution scenarios was carried out in order to evaluate their CO2 abatement potentials.
Zusammenfassung
Diese Publikation ist eine gekürzte Veröffentlichung aus dem Programm der PRES 2016 Konferenz. Obwohl alternative Prozesse wie Corex® oder Finex® bis zur industriellen Reife entwickelt wurden, hat die Hochofenroute weiterhin die größte Bedeutung aller Verfahren zur Herstellung von Roheisen. Aus diesem Grund wurden in den letzten Jahren eine Reihe von entsprechenden Modellen zur Prozessbeschreibung eingeführt. Zur analogen Beschreibung des Hochofenprozesses hat sich darüber hinaus das sogenannte Rist Diagramm etabliert. Ziel dieser Arbeit ist die Entwicklung eines umfassenden Hochofenmodelles für die Prozesssimulationsplattform gPROMS ModelBuilder®. Dieses Modell soll die funktionalen Zusammenhänge zwischen dem eigentlichen Reduktionsprozess, den Bedingungen in der Wirbelzone vor den Blasformen und den thermodynamischen Verfahrensbedingungen in einem mathematischen Modell verbinden. Zur Darstellung der Anwendungsmöglichkeiten wurden zum Beispiel eine Reihe von Strategien zur Verminderung des Koksverbrauches mit Hilfe des erstellten Hochofenmodelles analysiert, um deren Potential zur Minderung des CO2 Ausstoßes zu bewerten.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The blast furnace process is the most important iron making process worldwide. It has been in use for centuries and is still being developed in order to increase efficiency as well as productivity. In addition to this, the development of alternative iron making processes, so called smelting reduction processes, started in the 1970s. The main target of these efforts was to derive process routes capable of producing hot metal (HM) at low capacity using non-coking coal directly thereby reducing environmental emissions [1]. As of today, only the Corex® and the Finex® smelting reduction processes have been established on industrial scale.
Driven by economical as well as ecological reasons, blast furnace iron making significantly advanced in the past decades as well. Due to the introduction of numerous technological improvements such as higher blast temperatures, elevated top gas pressure levels, enhanced burden distribution, and sinter quality as well as the injection of hydrocarbons like coal, oil and natural gas, the overall reducing agent demand was successfully reduced to levels below 500 kg/t HM [2]. These developments contribute to the competitive efficiency of modern blast furnaces. Worldwide market shares of over 99% in recent years confirm that the blast furnace route remains the primary process to produce hot metal [3].
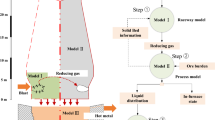
A schematic overview of the blast furnace process is illustrated in Fig. 1. In general, the blast furnace process can be characterized as a counter current, multi-phase heat and mass exchange reactor. Oxygen is removed from oxidic burden constituents and transferred to an ascending hot reducing gas phase which in turn transfers heat to the descending burden and coke [4]. Both the sensible heat as well as chemical reduction potential (through reducing gas) required for the blast furnace process are provided by the gasification of coke and hydrocarbons inside the raceway in front of blast furnace tuyeres. A way to characterize the conditions in the raceway is by means of the raceway adiabatic flame temperature (RAFT) [5]. This adiabatic temperature is a theoretical concept commonly considered in practice as an empiric parameter for a stable blast furnace operation [4].
Due to the importance of the blast furnace process, many different mathematical models have been developed in the past decades. These include statistical models, thermodynamic models as well as kinetic-dynamic models. While statistical models are based on a long term analysis of operation data, thermodynamic and kinetic models are based on mass and energy balances as well as chemical reaction kinetics [6]. All of these types of models are still being used in today’s iron making industry.
Another way of characterizing the blast furnace operation is provided by means of the Rist operating diagram (see Fig. 2). It is based on plots of reduction data of a lab-scale blast furnace [7] and was developed to a full-fledged analogue diagram in the following years. The distinctive feature of this operating diagram is the graphical representation of balances of carbon, oxygen, and hydrogen through an operation line. These elements are involved in the formation and utilization of the reducing gas mixture in a blast furnace [8]. In addition to this, the equilibrium conditions of involved indirect iron oxide reduction reactions are taken into consideration by means of an equilibrium line [9].
2 Modelling
The blast furnace model consists of three distinct sub topologies or calculation layers. The task of the main sub topology layer is the depiction of the material flows of the blast furnace process. The chemical and physical conversions are implemented using a black box model. In recent years, multiphase equilibrium calculation routines have already been developed for the comparable melter gasifier model in gPROMS® [10]. However, due to observed deviations regarding manganese and silicon contents in hot metal and slag, this approach was rejected for the blast furnace model. Hence, empiric elemental assignments and distribution coefficients of affected elements are used for an implicit implementation of blast furnace conversions and reactions. The connection between the sub models of the main calculation layer is established by material streams which represent material flow regimes occurring in the blast furnace process (see Fig. 3).
The second calculation layer is used to depict the gasification conditions at the blast furnace tuyeres. This task is assigned to the adiabatic flame temperature sub model. The third calculation layer is used to determine the overall thermodynamic conditions of the blast furnace process by making use of a specifically developed Rist sub model.
The adiabatic flame temperature sub model is used for the calculation of the RAFT. It can be derived by means of empiric formulas or very accurately from adiabatic mass and heat balance calculations [4]. The model presented in this work is based on the latter approach. Complete gasification under adiabatic conditions is performed with the hot blast and injectants entering the system boundary of the theoretical blast furnace tuyeres. The temperature of the resulting gas is equal to the RAFT.
The Rist sub model is required to construct the Rist operating diagram. Assuming ideal behaviour, it serves as the graphical representation of the blast furnace operation. This includes the depiction of the influences of varying operation parameters, such as blast temperature, burden pre-reduction as well as injection of hydrocarbons on the blast furnace process [8]. Thereby it is possible to use this analogue model for the interpretation and comprehension of different operation scenarios, while it also allows qualitative prediction of thermochemical changes in a blast furnace [11].
In general, the tasks of the calculation layers are carried out in parallel on the same hierarchical level as illustrated in Fig. 4. All three layers add up to an overall sub topology of the blast furnace model. Due to this combined modelling approach, the results of the adiabatic flame temperature sub model and the Rist sub model are indirectly excreting influence on the mass and energy balance of the black box model. Therefore, the interdependencies between blast furnace process, RAFT, and thermodynamic conditions can be described within the single mathematical model of the blast furnace unit operation model.
3 Simulation Results
The developed model has successfully been validated against plant data as well as established calculation tools. In addition to this, several parameter and input variation scenarios have been investigated including pre-reduced iron carrier usage and natural gas injection [3]. The simulation presented in this work illustrates the effects of coke substitution by means of hydrogen injection. A necessary assumption for this scenario is the constant thermodynamic efficiency, which is expressed by a constant horizontal approach of the Rist operation line to the wustite equilibrium. Depending on the operating conditions, the blast furnace gas temperature can range from 100 to 230 °C [2] In this particular case, it is specified with 150 °C. Based on this, the injection of H2 is increased from 0 to 200 m3 (STP)/t (HM).
The overall simulation results of this variation are given in Figs. 5, 6 and 7. It can be observed in Fig. 5 that the specific amount of coke required for the process drops from 455 to 401 kg/t HM. Moreover, the specific amount of the hot blast also decreases from 952 to 889 m3 (STP)/t HM. This effect is explained by a shift in the reduction mechanisms in the blast furnace, which is caused by the hydrogen injection. Since solid carbon is exchanged by gaseous H2, the highly endothermic direct reduction with solid carbon is partially replaced by a less endothermic indirect reduction with H2. Therefore, the input of the energy-rich hot blast has to be reduced in order to ensure a constant blast furnace gas temperature. Fig. 6 illustrates calculated process outputs. A minor change can be observed for the specific slag production rate with a decrease from 222 to 217 kg/t HM. In addition to this, the CO2 equivalent emissions decrease from 1428 to 1259 kg/t HM, which is equal to a reduction of approximately 12%. Both effects are linked to the decreasing coke mass flow which supplies both carbon and slag forming components to the blast furnace process. A drop from 2300 to 2022 °C is visible for the RAFT, which is explained by the decreasing supply of the hot blast and the increasing hydrogen injection rate.
Fig. 7 depicts the corresponding Rist operating diagram of this simulation. The gradient of the operation line is decreasing with increasing hydrogen injection. This can be translated to a decreasing reducing agent demand of the process which is in line with the observed decrease of CO2 equivalent emissions. Furthermore, a drop of direct reduction percentage can be observed which corresponds to the shift in the reduction mechanisms from carbon to hydrogen.
4 Conclusions and Outlook
The model described in this work links the basic mass and energy balances of the blast furnace with an additional model for the raceway adiabatic flame temperature as well as an analogue process description offered by the Rist operating diagram. This allows for a variety of possible applications including the verification and benchmarking of the existing as well as predictive simulation of new blast furnace process set-ups. Moreover, it is possible to study alternative process scenarios like the usage of pre-reduced iron carriers or low-emission scenarios like natural-gas or hydrogen injection. The results presented in this work demonstrate the considerable potential of such theoretical approaches to reduce CO2 emissions of blast furnace processes.
Change history
30 March 2020
<Emphasis Type="Italic">The provided reference to the original publication, which was the basis for this article, was incomplete. The acknowledgements of this article should be amended as follows: We acknowledge the permission to re-publish a modified version of the original article (Spanlang A., Wukovits W., Weiss B., Development of a Blast Furnace Model with Thermodynamic Process Depiction by Means of the Rist Operating Diagram, Chemical Engineering Transactions, 52 (2016), pp 973–978) based on a contribution to</Emphasis> the 19th Conference on Process Integration, Modelling and Optimisation for Energy Saving and Pollution Reduction – PRES 2016 (27–31 August, 2016, Prague, Czech Republic).
References
Flickenschild, J.; Hauk, R.; Steffen, R.: Iron, 4. Smelting Reduction Processes, in: Ullmann’s Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH GmbH & Co. KGaA, 2012
Lüngen, H. B.; Yagi, J.-I.: Iron, 2. Blast Furnace Process, in: Ullmann’s Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH GmbH & Co. KGaA, 2012
Spanlang, A.: Modelling of the Blast Furnace Route in gPROMS®, Master’s Thesis, Institute of Chemical, Environmental and Bioscience Engineering (ICEBE), Wien: TU Wien, 2015
Geerdes, M.; Toxopeus, H.; van der Vliet, C.: Modern Blast Furnace Ironmaking—an introduction, Amsterdam: IOS Press BV, 2009
Peacey, J. G.; Davenport, W. G.: The Iron Blast Furnace—Theory and Practice, Oxford: Pergamon Press, 1979
Bogdandy, L. v.; Engell, H.-J.: Die Physikalische Chemie der Eisen und Stahlerzeugung – Vorträge einer vom Verein Deutscher Eisenhüttenleute zusammen mit dem Haus der Technik, Essen, durchgeführten Berichtsreihe, Düsseldorf: Verlag Stahleisen m.b.H., 1967
Rist, A.; Bonnivard, G.: Etude Experimentale de la reduction d’agglomeres de Minerais de fer par un gaz a contre-courant, Revue de Métallurgie, 95 (1962), pp 401–415
Rist, A.; Meysson, N.: A Dual Graphic Representation of the Blast-Furnace Mass and Heat Balances, Journal of Metals, 19 (1967), pp 50–59
Babich, A.; Senk, D.; Gudenau, H. W.; Mavromatis, K. Th.: Ironmaking—Textbook, Aachen: RWTH Aachen, 2008
Almpanis-Lekkas, O.; Weiss, B.; Wukovits, W.: Development of an Industrial Iron-Making Melter Gasifier Model with Multiphase Equilibrium Calculations, Chemical Engineering Transactions, 39 (2014), pp 823–828
Kundrat, D. M.; Miwa, T.; Rist, A.: Injections in the Iron Blast Furnace: A Graphics Study by Means of the Rist Operating Diagram, Metallurgical Transactions B, 22B (1991), pp 363–383
Acknowledgements
The authors gratefully acknowledge the funding support of K1-MET GmbH, metallurgical competence center. The research program of the K1-MET competence center is supported by COMET (Competence Center for Excellent Technologies), the Austrian program for competence centers. K1-MET is funded by the Federal Ministry for Transport, Innovation and Technology, the Federal Ministry for Digital and Economic Affairs, the provinces of Upper Austria, Tyrol and Styria as well as the Styrian Business Promotion Agency (SFG).
Funding
Open access funding provided by TU Wien (TUW).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Spanlang, A., Wukovits, W. & Weiss, B. Development of a Blast Furnace Model with Thermodynamic Process Depiction by Means of the Rist Operating Diagram. Berg Huettenmaenn Monatsh 165, 243–247 (2020). https://doi.org/10.1007/s00501-020-00963-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00501-020-00963-6