Abstract
The objectives of this research were to contrast the expression values of heat shock protein (HSP70) and interleukins 2, 6 and 12 (IL 2, IL 6 and IL 12) genes in summer and winter in two different locations in Egypt (Alexandria zone and Matrouh zone) to deduce changes in thermo-physiological traits and biochemical blood metabolites of Barki sheep. A total of 50 ewes (20 in Alexandria and 30 in Matrouh) were individually blood sampled to determine plasma total protein (TP), Albumin, Globulin and Glucose constituents and T3, T4 and cortisol hormones. The thermo-physiological parameters of rectal temperature (RT, °C), skin temperature (ST, °C), Wool temperature (WT, °C), respiration rate (RR, breaths/min) and pulse rate (PR, beats/min) were measured for each ewe. Expressions of IL 2, IL 6, IL 12 and HSP 70 in summer and winter were analyzed along with thermo-physiological parameters and blood biochemical metabolites. In both locations, THI had significant effects on ST, WT, PR and RR, but not significant on RT. However, it had no significant effects on blood plasma metabolites and hormonal concentrations in the two locations in summer and winter. In Alexandria location, THI had negative significant effect on the expressions of IL-2 and IL-6 but positively affected on HSP70 genes in winter, while the expression of IL-12 gene was not affected by seasons, whereas in Matrouh zone, the effects of THI on the expressions of all tolerance genes were not significant. The results of the current study suggest that IL-2, IL-6 and HSP70 genes could be used as molecular markers for heat/cold stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The conditions under which an animal is exposed to sudden changes in the ambient meteorological elements that cause its inability to cope with the endemic environment or failure to achieve prosperity according to its genetic potential are known as climatic stress (Dobson and Smith 2000; Sunil Kumar et al. 2011). In addition to climatic stress, animals could be subjected to other types of stressors such as production, transportation and immune stresses (Mirkena et al., 2010). The ability of an animal to respond to a particular stressor depends on history of previous exposure to adverse circumstances, genetic make-up, age, season and physiological status (Blecha et al. 1983; Mason et al. 1991). The recent phenomenon of global warming causes heat stress to become one of the principal factors that imposes negative impacts on production and reproduction in farm animals (Silanikove et al. 1987). Variation in ambient temperature either above or below the upper or lower critical temperature leads to a condition known as the thermal stress (Pandey et al. 2014). Thermal stress may compromise reproductive efficiency of farm animals in both sexes and hence affect negatively milk, meat, wool production, feed intake, growth rate, immunity and cause changes in blood constituents and most of the biological pathways (Maibam et al. 2018; Skibiel et al. 2018; Becker et al. 2020). To overcome the adverse effect of heat stress caused by global warming, adaptation to hot climate practiced on livestock species became highly imperative in order to increase the adaptive capacity of animals. This involves different physiological pathways, biochemical routes, compositional alterations and hormonal changes to help animals to survive and produce under prevailing climatic conditions (St Pierre et al. 2003). Tolerance or susceptibility to thermal stress varies among animals, but can be explored at the DNA level to identify superior animals that carry genes of adaptability traits, in order to be used in selection programs to improve animal tolerance to heat stress (Singh et al. 2017).
Heat shock proteins (HSP) are a family of proteins that are produced by cells in response to exposure to stressful conditions (Park et al. 2007; Singh et al. 2017). They play essential roles in the cellular homeostasis. HSPs have been classified based on their molecular weights to HSP90 (85–90 kDa), HSP70 (68–73 kDa), HSP60, HSP47, and small HSPs (12–43 kDa) (Park et al. 2007; Singh et al. 2017). One of the most abundant and the best characterized is the HSP70 (Banerjee et al. 2014). These proteins are induced by stress and play essential roles in environmental stress tolerance and thermal adaptation (Banerjee et al. 2014; Garbuz, and Evgen’ev 2016; Singh et al. 2017). Moreover, Interleukin (IL) are a group of cytokines formed of secreted proteins and signal molecules that were first seen to be expressed by leukocytes as IL2, IL6, and IL12 (Bharati et al. 2017). They play a significant role in promoting the immune system and establishing the balance between humoral Th2 and cell mediated Th1 responsiveness (Bharati et al. 2017). In order to determine superior animals that carry the genes of adaptability traits, the current study performed an expression analysis for HSP70, IL2, IL6 and IL12 genes in Barki sheep in summer and winter seasons in two distinct locations (Alexandria zone vs Matrouh zone) in relation to changes in thermo-physiological parameters and biochemical blood components under heat stress and thermoneutral conditions.
Material and methods
Animals and locations
The studied animals belonged to Barki sheep breed. The origin of Barki is North Africa in the coastal Mediterranean zone, and characterized by its medium size, light colour and well adapted to survival in the hot arid environment (Abdel–Moneim et al. 2009; Abousoliman et al. 2020), which help to increase heat loss by evaporation while absorbing less heat (Berihulay et al. 2019).
Data were collected from The Sustainable Development Center for Matrouh Resources—Desert Research Center, Matrouh (31° 21′ 11ʺ N, 27° 11′ 05ʺ E) and Animal Production Research Station- Faculty of Agriculture—Alexandria University – Alexandria (31° 12′ 43ʺ N, 29° 59′ 02ʺ E). A total of 50 Barki ewes (30 in Matrouh and 20 in Alexandria) ageing 3–4 years were used in this study. The experiments were carried out in two distinct time spans coinciding with the coldest and hottest periods of year viz. mid-winter (January) and mid-summer (August). The animals were raised under similar management conditions and were monitored throughout the experimental period.
Blood samples and biochemical parameters
Two blood samples each of 5 ml were collected from each ewe (The samples were taken in the mid of August and mid of January), via jugular vein puncture into a heparinized vials treated with 0.5 ml of 2.75% EDTA (Pspark, UK), as anticoagulant and were transferred immediately to the manipulation laboratory in an ice box for biological and molecular tests. Plasma was analyzed to determine the total protein (TP), Albumin, Globulin and Glucose calorimetrically by Hitachi 901 spectrophotometer and using STANBIO commercial kits. Blood cortisol, triiodothyronine (T3) and thyroxine (T4) concentrations were determined by ELISA method using commercial kits (cortisol, T3 and T4 ELISA monobind Inc., Lake Forest, CA, USA).
Physiological parameters
At the day of blood sampling, the thermo-physiological parameters of rectal temperature (RT, °C), skin temperature (ST, °C), wool temperature (WT, °C), respiratory rate (RR, breaths/min) and pulse rate (PR, beats/min) were collected from individual ewes early in the morning (7:00–8:00 am) before access to feed and in the afternoon (2:00–3:00 pm).
Temperature-humidity index (THI)
Data on meteorological variables, ambient temperature (AT) and relative humidity (RH) were obtained mid of August when AT is usually the highest and mid of January when it is the lowest to represent summer and winter seasons. THI was calculated according to the formula: THI = 0.8 × AT + [(RH % ÷ 100) × (AT—14.4)] + 46.4 (Kendall and Webster 2009). The obtained values of THI in Alexandria and Matrouh areas were 81.73 and 80.72 in summer, respectively, while the corresponding values in winter were 60.79 and 65.20, indicating that the experimental animals were under thermoneutral conditions in winter, but suffered from heat stress in summer.
Total RNA extraction and cDNA preparation
Total RNA was extracted from the blood tissue using total RNA Extraction kit (Total RNA Purification Mini Spin Kit), following the standard protocol of the manufacturer. Quality and quantity of RNA samples were determined by BioDrop (BioDrop µLite, UK). RNA samples giving an optical density (O.D) between 1.8 and 2.0 should contain pure RNA without protein contamination, therefore were utilized in further analysis. Total isolated RNA was treated with DNase I using RNeasy Mini Kit and integrity was determined by 1.5% denaturation agarose gel electrophoresis prior to cDNA synthesis. Total RNA was converted to cDNA using ProtoScript First Strand cDNA synthesis kit (Biolab, UK) following the manufacturer protocol. The prepared cDNA was analyzed using PCR and stored at − 80 °C until further use for qRT-PCR technique.
Quantitative real-time PCR (qRT-PCR)
Real time-PCR was performed to amplify target and reference genes on Light Cycler 480 (Roche) with Eva Green q.PCR reaction kit (Qarta). It was carried out using the SYBR green following manufacturer instructions. The primers were designed using primer 3 software from NCBI database (Table 1) and the Ensembl (EMBL-EBL Wellcome Trust Sanger Institute, Cambridegeshire, UK). The real-time PCR program was as follows: initial denaturation at 95 °C for 15 min; 40 cycles of 94 °C for 15 s; annealing at 56 °C for 30 s and extension at 72 °C for 30 s. Expression of GAPDH was taken as an endogenous reference (Migaud et al. 2005). Relative quantification of target genes was done by 2^ (−ΔΔCt) method (Livak and Schmittgen 2001).
Statistical analysis
Generalized linear model (GLM) was utilized to test the effect of locations (Alexandria city vs Matrouh city), season (summer vs winter) using SAS software (SAS, 2012) according to the following model:
Yijk = µ + Li + Sj + eijk where,
Yijk: either gene expression profile, biochemical blood components parameters or physiological parameters; µ: an underlying constant specific to each trait; Li: the effect of ith location (i = 1 and 2); Sj: the effect of jth season (j = 1 and 2) and eijk: random errors assumed to be independent normally. Results were presented as means ± SE of a minimum of two independent replicates. Duncan’s multiple range test checked the significance (P < 0.05) of the differences between group means (Table 2).
Results
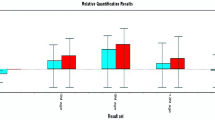
Differences between winter and summer seasons for thermo-physiological parameters, blood plasma metabolites and average expression of tolerance genes underlying the ability of Barki ewes to withstand meteorological changes in Alexandria and Matrouh areas are presented in Tables 3 and 4. Changes in THI values from winter to summer had no effect on RT in Alexandria and Matrouh areas. RT of ewes in experimental flocks were consistent at about 39 °C with a range of differences not exceeding 0.1 °C between seasons and between locations. However, ST, WT, PR and RR but not RT were different between seasons in both locations. These thermo-physiological parameters were higher in summer heat stressed ewes compared to those under winter thermoneutral conditions. Seasonal effects on blood plasma metabolites and hormonal concentrations were not significant in both experimental locations. Seasonal effects on expressions of HSP 70, IL-2 and IL-6 but not on IL-12 genes were significant in Alexandria area, showing upregulation in summer compared to winter. While the upregulation of HSP 70 gene expression was detected in Alexandria in winter rather than in summer. In Matrouh area, however, none of the tolerance genes showed significant changes in expression by season but were arbitrarily upregulated in either season.
Discussion
Physiological parameters
Sheep are homoeothermic animals. They maintain body temperature balance by dissipation of the excessive heat outside their body mass through number of biological mechanisms. These biological mechanisms are increasing respiration rate, panting and flushing heat from the body surface to the ambient environment through the skin by conduction, convection, radiation or evaporation (El-Zeiny 2011; Berihulay et al. 2019). Thus, RT is used as an indicator for animal core heat and increases only when body fails to maintain heat balance (Berihulay et al. 2019). Therefore, the consistency of ewes RT in the current study in both locations confirmed the success of these animals to preserve body temperature at the normal level regardless of the increase in THI values above 80 especially in summer in both locations. However, the increase of thermo-physiological parameters of ST, WT, PR and RR in summer when THI increases above 80 verified the commencement of the physiological activities to get rid of the excessive heat. These parameters exhibit an immediate response to the climatic stress and, therefore, provide an evidence for the optimum level of comfort/discomfort level to the animal.
The ability of an animal to withstand the discomfortable thermal conditions is determined by the observed changes in the physiological parameters. Al-Haidary et al. (2012) obtained high average ST value of 38.13 °C in Najdi sheep in the hot Saudi Arabian summer season and Rathwa et al. (2017) found significant increase in ST of Indian sheep from 35.5 °C in winter to 37.8 °C in summer. The high ambient air temperature causes increase of ST as a result of redistribution of blood flow towards the body surface and therefore increase the skin blood flow to transfer heat outside the body ending with regulation of heat between body core and skin (Indu et al. 2014; Marai 2007).
The cardio-respiratory system is influenced by season, day timing, ambient temperature and relative humidity. The first mechanism taken by an animal subjected to heat stress is the increment in RR, which causes loss of heat through evaporation (Renaudeau et al. 2012). Furthermore, Ribeiro et al. (2014) demonstrated that stressed animals utilize respiratory mechanism to avoid increase in RT and maintain homeostasis. Heat stress eventually leads to dissipation of moisture by evaporation from the respiratory tract to maintain thermal balance. These mechanisms are very crucial in preventing hypothermia. Furthermore, Nienaber et al. (2007) revealed that the animal tries to maintain homeostasis by wasting heat load from the body.
In accordance with the current results, Rathwa et al. (2017) reported an increase in RR from 40 breaths/min in winter to 108 in summer and Okoruwa (2014) obtained an increase in RR of African black dwarf goats from 16.04 breaths/min under thermoneutral conditions to 23.01 breaths/min. Moreover, Adedeji (2012) found significant increase in RR of black West African Dwarf Goats compared to white goats from 60.4 breaths/min for to 65.58 breaths/min, which confirmed that black goats are less tolerant to the ambient environmental temperature.
The genetic make-up of sheep influences their response to heat stress measured by change in RR. Joy et al. (2020) reported that Dorper lambs showed less increase in RR than Merino second cross lambs (Poll Dorset × Merino/Border Leicester). Also, variation in breed response to heat stress in goats was apparent (Rout et al. 2018). Comparison between RR of Jamunapari and Barbari goats indicated that RR of the two breeds were 37 and 45 under stress while were 30 and 33 under thermoneutral conditions.
In general, exposure of animals to heat stress causes alterations in circadian rhythm of the cardiac functions including increasing PR which will increase the blood flow from the core to the peripheral of the body. Consequently, previous changes cause higher heat loss by conduction, convection, radiation and water loss by diffusion through the skin (Marai et al. 2007). The increase in PR was found to be positively correlated with RR (Popoola et al. 2014). The capacity of the animal to accommodate the cardiac function alteration related to tolerance to thermal stress was found to be influenced by the genetic make-up and breed differences. For example, coat color of African dwarf goats had a direct effect on PR. During heat stress, PR of black goats counted 83.57 beats/min while white goats recorded 74.97 beats/min (Adedeji 2012). Also, Rout et al. (2018) found that variation in PR of Jamunapari and Barbari goat breeds were significantly high in peak heat stress period recording 111 and 125 beats/min in hot season and 99 vs 108 beats/min under thermoneutral conditions in the two breeds respectively.
Blood analysis
The non-significant effects of THI values in summer and winter seasons on blood biochemical components in either location indicated that Barki sheep breed succeeded to express high capability to maintain the body biological functions at normal levels. This may be associated with the breed competence to regulate body temperature and maintain it at the normal level to reduce the adverse effect of heat stress of summer by increasing PR, RR, ST and WT (Nienaber et al. 2007; Renaudeau et al. 2012; da Silva et al. 2017).
Relative expression of HSP 70, IL 2, 6 and IL12 genes
In both experimental zones, Barki sheep were under heat stress in summer. The non-significant differences between all expressions of tolerance genes in winter and summer of Matrouh area associated with significant differences for the same genes except IL 12 indicated different response of animals to seasonal variations probably due to different ambient climatic conditions between locations. The upregulation of IL 2 and IL 6 in summer concomitant with upregulation of HSP70 in winter indicated different mode of action for the two types of genes. In Alexandria, HSP70 can be used as a molecular marker for cold adaptation, while IL 2 and IL 6 genes can be used for the same purpose in summer. IL12 has no impact on climatic conditions but more research may be needed to explore other possible roles of the gene.
Similar results by Bharati et al. (2017) illustrated the putative role of IL2 and IL6 as tolerance genes against heat stress; performing the mRNA expression analysis during short- and long-term heat stress acclimation in Tharparkar cattle showed significant increase in IL2 and IL6 expressions after exposure of animals to temperature of 42 °C for 6 h daily for a long period of 23 days. IL2 and IL6 could possibly play a key role to elicit the immune response to ameliorate the thermal insults during long-term heat stress acclimation. However, mRNA expression of IL2 and IL6 genes in peripheral blood was higher in mid-winter than summer for all of three age groups of tropical and temperate goat breeds suggesting an essential role of IL2 and IL6 genes in modulation of cold stress by maintaining cellular homeostasis (Maurya et al. 2013).
HSP70 is a molecular chaperone that plays a crucial role in biological process of protein. It was found to be the most temperature sensitive gene correlated with thermotolerance (Park et al. 2007; Singh et al. 2017). The role of this gene was demonstrated in goats by the expression analysis to be effective in cold adapted than in heat-adapted breeds in both summer and winter (Banerjee et al. 2014). This role and expression of HSP70 was different among breeds of sheep in arid and semi-arid areas and more expressed in winter (Singh et al. 2017). Moreover, the relative expression of HSP70 gene in skin of Tharparkar and Karen fares cattle during different seasons were high in summer and winter than in spring, indicating that this gene works under adverse cold or hot thermal stress.
Conclusion
Barki sheep are well adapted to harsh desert environment. They have high tolerance capacity to withstand heat stress by altering several physiological mechanisms such as increasing ST, WT, PR and RR while maintaining normal blood plasma constituents. Also, IL-2, IL-6 and HSP 70 are temperature sensitive genes. Their expressions could be used as molecular marker for heat/cold stress.
Data availability
Available upon request.
Code availability
Available upon request.
References
Abdel-Moneim AY, Ahmed AM, Ibrahim MM, Mokhtar MM (2009) Flock dynamics of desert Barki sheep in relation to age structure. Trop Anim Health Prod 41:899–905. https://doi.org/10.1007/s11250-008-9277-4
Abousoliman I, Reyer H, Oster M, Muráni E, Mourad M, Rashed MAS, Mohamed I, Wimmers K (2020) Analysis of candidate genes for growth and milk performance traits in the Egyptian Barki sheep. Animals 10(2):197. https://doi.org/10.3390/ani10020197
Al-Haidary AA, Aljumaah RS, Alshaikh MA, Abdoun KA, Samara EM, Oka AB, Aluraiji MM (2012) Thermoregulatory and physiological responses of Najdi sheep exposed to environmental heat load prevailing in Saudi Arabia. Pak Vet J 32:515–519
Banerjee D, Upadhyay RC, Chaudhary UB, Kumar R, Singh S, Ashutosh GJM, Polley S, Mukherjee A, Das TK, De S (2014) Seasonal variation in expression pattern of genes under HSP70: seasonal variation in expression pattern of genes under HSP70 family in heat- and cold-adapted goats (Capra hircus). Cell Stress Chaperones 19(3):401–408. https://doi.org/10.1007/s12192-013-0469-0
Berihulay H, Abied A, He X, Jiang L, Ma Y (2019) Adaptation mechanisms of small ruminants to environmental heat stress. Animals 9:75. https://doi.org/10.3390/ani9030075
Bharati J, Dangi SS, Mishra SR, Chouhan VS, Verma V, Shankar O, Bharti MK, Paul A, Mahato DK, Rajesh G, Singh G, Maurya VP, Bag S, Kumar P, Sarkar M (2017) Expression analysis of Toll like receptors and interleukins in Tharparkar cattle during acclimation to heat stress exposure. J Therm Biol 65:48–56. https://doi.org/10.1016/j.jtherbio.2017.02.002
Blecha F, Pollmann DS, Nichols DA (1983) Weaning pigs at an early age decreases cellular immunity. Anim Sci J 56(2):396–400. https://doi.org/10.2527/jas1983.562396x
Dobson H, Smith RF (2000) What is stress and how does it affect reproduction? Anim Reprod Sci 60:743–752. https://doi.org/10.1016/S0378-4320(00)00080-4
El-Zeiny WT (2011) Effects of season, housing environment and water deprivation on rectal and skin temperature regulation in Barki desert sheep. Journal of Animal and Poultry Production, Mansoura University 2:411–426. https://doi.org/10.21608/JAPPMU.2011.83404
Garbuz DG, Evgen’ev MB, (2016) The evolution of heat shock genes and expression patterns of heat shock proteins in the species from temperature contrasting habitats. Russ. J. Genet 53(1):21–38. https://doi.org/10.1134/S1022795417010069
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−deltadeltaCt method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Maibam U, Hoodaa OK, Sharmab PS, Upadhyaya RC, Mohanty AK (2018) Differential level of oxidative stress markers in skin tissue of zebu and crossbreed cattle during heat stress. Livest Sci 207:45–50. https://doi.org/10.1016/j.livsci.2017.11.003
Marai IFM, El-Darawany AA, Fadiel A, Abdel-Hafez MAM (2007) Physiological traits as affected by heat stress in sheep-a review. Small Rumin Res 71:1–12. https://doi.org/10.1016/j.smallrumres.2006.10.003
Migaud M, Daveau A, Malpaux B (2005) MTNR1A melatonin receptors in the ovine premammillary hypothalamus: day-night variation in the expression of the transcripts. Biol Reprod 72:393–398. https://doi.org/10.1095/biolreprod.104.030064
Mirkena T, Duguma G, Haile A, Tibbo M, Okeyo AM, Wurzinger M, Sölkner J (2010) Genetics of adaptation in domestic farm animals. Livest Sci 132:1–12. https://doi.org/10.1016/j.livsci.2010.05.003
Pandey V, Nigam R, Saxena A, Singh P, Sharma A, Swain DK, Sharma L, Dixit S (2014) Influence of season on biochemical attributes of Bhadawari buffalo bull semen: effect of temperature and humidity. Indian J. Anim. Res 4(2):201–209. https://doi.org/10.5958/2277-940X.2014.00006.0
Park H, Ahn IY, Lee HE (2007) Expression of heat shock protein 70 in the thermally stressed Antarctic clam Laternula elliptica. Cell Stress Chaperones 12(3):275–282. https://doi.org/10.1379/csc-271.1
St Pierre NR, Cobanov B, Schnitkey G (2003) Economic loss from heat stress by US livestock industries. Int J Dairy Sci 86:52–77. https://doi.org/10.3168/jds.S0022-0302(03)74040-5
Becker CA, Collier RJ, Stone AE (2020) Physiological and behavioral effects of heat stress in dairy cows-invited review. Int. J. Dairy Sci. 103, In press. https://doi.org/10.3168/jds.2019-17929
Mason WA, Mendoza SP, Moberg GP (1991) Persistent effects of early social experience on physiological responsiveness. Primatology today, 469–471
Silanikove N (1987) Effect of imposed reduction in energy intake on resting and fasting heat production in the black Bedouin Goat. Nutr. Rep. Int. 35: 725–731.https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=8252154
Skibiel AL, Zachut M, do Amaral BC, Levin Y, Dahl GE (2018) Liver proteomic analysis of postpartum Holstein cows exposed to heat stress or cooling conditions during the dry period. Int. J. Dairy Sci 101, 705–716. https://doi.org/10.3168/jds.2017-13258
Sunil Kumar BV, Kumar A, Kataria M (2011) Effect of heat stress in tropical livestock and different strategies for its amelioration. J. Stress Physiol. Biochem. 7 (1), 45–54.https://agris.fao.org/agris-search/search.do?recordID=RU2012103284
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement of animal rights
Animal care and use were conducted in accordance with the guidelines of Alexandria University, Egypt. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Alexandria University (ALEXU-IACUC no. AU08190115108).
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rawash, R.A.A., Sharaby, M.A., Hassan, G.ED.A. et al. Expression profiling of HSP 70 and interleukins 2, 6 and 12 genes of Barki sheep during summer and winter seasons in two different locations. Int J Biometeorol 66, 2047–2053 (2022). https://doi.org/10.1007/s00484-022-02339-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-022-02339-6