Abstract
A versatile meteorological index for predicting heat stress in dairy cattle remains elusive. Despite numerous attempts at developing such indices and widespread use of some, there is growing skepticism about the accuracy and adequacy of the existing indices as well as the general statistical approach used to develop them. At the same time, precision farming of high-yielding animals in a drastically changing climate calls for more effective prediction and alleviation of heat stress. The present paper revisits classical work on human biometeorology, particularly the apparent temperature scale, to draw inspiration for advancing research on heat stress in dairy cattle. The importance of a detailed, mechanistic understanding of heat transfer and thermoregulation is demonstrated and reiterated. A model from the literature is used to construct a framework for identifying and characterizing conditions of potential heat stress. New parameters are proposed to translate the heat flux calculations based on heat-balance models into more tangible and more useful meteorological indices, including an apparent temperature for cattle and a thermoregulatory exhaustion index. A validation gap in the literature is identified as the main hindrance to the further development and deployment of heat-balance models. Recommendations are presented for systematically addressing this gap in particular and continuing research within the proposed framework in general.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overview
Heat stress in dairy cattle has been the subject of continued interest for several decades. A significant portion of the literature can be categorized as attempts to develop heat stress indices through regression analysis of meteorological parameters such as ambient temperature, humidity, wind speed, and solar radiation and animal responses such as body temperature, respiration rate, and milk yield, or select the “appropriate” index from the literature. In the latter case, meteorological data are used to evaluate various available indices, in search of indices that show significant statistical correlation with animal response data. Despite numerous attempts, a versatile index remains elusive as evidenced by continual revisions to existing indices and development of new indices. A recent review (Ji et al. 2020a) lists as many as 20 heat stress indices for dairy cattle.
An alternative, possibly complementary, approach is to use mechanistic models of heat generation and dissipation to identify conditions of potential stress based on the heat balance of the animal. Examples include the work of McArthur (1987), Ehrlemark and Sällvik (1996), Turnpenny et al. (2000a, b), McGovern and Bruce (2000), and Thompson et al. (2014). Despite its fundamental robustness, the heat-balance approach has attracted much less attention than the statistical approach and its application remains limited. Very few studies deal with the application and assessment of heat-balance models, including for instance the papers by Bloomberg and Bywater (2007) and van der Linden et al. (2018). Even fewer attempts have been made at systematic application of a heat-balance model to identify conditions of potential heat stress and to develop relevant indices accordingly. The work of Berman (2004, 2005, 2006) is a notable exception, which nevertheless also resorts to linear regression of modeling results to develop simplified indices, and in some cases, linear fits stretched beyond the range of the underlying empirical data. The relative unpopularity of mechanistic models can be attributed to their formal complexity, large number of input parameters and the need for iterative solutions as well as lack of experimental data that can be used for reliable estimation of the parameters or validation of the models, especially for the modern high-production dairy cow.
The present paper is an attempt to outline a path forward based on mechanistic models for predicting conditions of potential heat stress in dairy cattle. The many statistical indices are not reviewed here. The reader is instead referred to the recent publications by Ji et al. (2020a) and dos Santos et al. (2021). In the absence of similar reviews of heat-balance models, the few existing models are briefly discussed here. Adopting classical theoretical work on human thermal comfort and perception, particularly the apparent temperature scale (Steadman 1984), two basic questions are examined: first, is the hitherto shortcoming of the conventional (statistical) approach methodological? Second, is there inspiration to be drawn from human biometeorology? More specifically, how can thermodynamic modeling and prediction of heat stress in cattle offer new perspectives and possibilities?
Thermoregulation
As homeotherms, cattle maintain a relatively constant core body temperature in a range known as the “thermoneutral” zone, where minimal energy is spent on thermoregulation and maximal energy is devoted to metabolism and production (Mount 1974; Godyń et al. 2019). Beyond this zone, thermoregulatory mechanisms are activated to return to the thermoneutral zone (dos Santos et al. 2021). There is a limit to the effectiveness of the thermoregulatory mechanisms beyond which thermal stress, hyperthermia or hypothermia, occurs (Kamal et al. 2016; Sejian et al. 2018). In this zone, behavioral changes such as reduced feed intake, increased water intake, and reduced lying time and seeking shade are triggered as secondary coping mechanisms, effectively assisting the physiological thermoregulatory responses (Ratnakaran et al. 2017; Madhusoodan et al. 2019). In thermodynamic terms, the response to heat stress can be divided into (1) increasing heat dissipation (total heat transfer coefficient), e.g. through enhanced perspiration and increased surface (skin) temperature, and (2) reducing the endogenous heat generation, e.g. by reducing feed intake and activity. Hyperthermia (heat stress) occurs when the heat dissipation cannot be adequately modulated to meet the thermoneutral heat generation and maintain the basal (normal) body temperature (Spiers 2012). For a detailed discussion of thermoregulation and thermal stress in cattle, see the review by dos Santos et al. (2021) and the sources therein cited.
Heat stress: indicators and predictors
As pointed out by West (2003), the term heat stress is used rather loosely to signify the climate, climatic effects, or the animal’s response. Alternative terminologies are also used in the literature, e.g. “heat load” by Heinicke et al. (2018), with the same meanings. Here, the definition put forth by Lee (1965) is adopted where heat stress means “the conditions that displace the animal’s thermoregulation system out of the thermoneutral zone,” and heat strain is accordingly “the displacement or deviation of the physiological, behavioral, or productive parameters from the corresponding base values in the thermoneutral zone.”
Heat strains are “indicators” of heat stress, i.e. signs that heat stress is occurring (e.g. increased respiration rate) or has already occurred (e.g. reduced milk yield). Direct reliance on such indicators for environmental control in livestock management would be challenging as it requires close, real-time monitoring of various physiological parameters and operation of the environmental control systems based on such observations. Adding to this challenge is the fact that some thermoregulatory responses (indicators) such as sweating or breathing tend to be highly variable from animal to animal, even within the same genotype or herd. See for instance the work of Maia et al. (2005a, b) and Gebremedhin et al. (2010) on dairy cattle and similarly the work of Gaughan et al. (2010) on beef cattle. Meteorological parameters, on the other hand, are readily available either from on-farm measurements or nearby weather stations. Furthermore, some indicators can only be observed when stress has already occurred [milk yield reduction (dos Santos et al. 2021)] or is well underway (core body temperature rise). Therefore, indices based on meteorological parameters, primarily ambient temperature, humidity, and wind speed, are sought to identify and categorize the conditions that are likely to cause heat stress. In this sense, a heat stress index serves as a “predictor.”
Despite more than 60 years of research, the observation by Berman (2005) that no clear criteria exist for conditions in which heat stress relief is needed remains the case. Most existing heat stress indices are applicable to temporally averaged, herd-level indicators. Even at this resolution, correlations are highly variable. See, for instance, the correlations of daily milk yield and milk temperature with ten different environmental indices presented in the paper by Ji et al. (2020b). Such levels of resolution and accuracy can be inadequate to address the needs of precision livestock farming in a rapidly and drastically changing climate.
As shown in a recent review (Ji et al. 2020a), the 40 years following the introduction of the temperature-humidity index [THI (Thom and Bosen 1959; Bianca 1962)] can be roughly summarized as readjustments and reformulations of essentially the same index (eight variants of THI and several similar indices). The alternative indices proposed in the last 20 years, e.g. the Comprehensive Climate Index [CCI (Mader et al. 2010)], are more complex in form, but were derived following the same general methodology, i.e. starting with some variant of THI and introducing incremental regression-based adjustments for air speed or solar radiation. See, for example, the development of the Heat Load Index [HLI (Gaughan et al. 2008)] and its expansion to CCI (Mader et al. 2010). Moreover, some of the adjustments demonstrate non-physical features. For example, the wind speed adjustments in the high-radiation variant of HLI (Gaughan et al. 2008) and CCI (Mader et al. 2010) have non-zero intercepts (respectively -11°C and +3°C at u=0) and non-zero slopes even at wind speeds as high as 25 m/s, i.e. non-asymptotic behavior.Footnote 1 Finally, the adjustments, particularly the wind-speed term in CCI (Mader et al. 2010), are rather complex in form.
More recently, indices such as the Dairy Heat Load Index [DHLI (Lees et al. 2018)] and the Equivalent Temperature Index for Cattle [ETIC (Wang et al. 2018)] have presented a breakaway from the practice of incremental improvements to THI, although they too rely almost exclusively on regression analyses of meteorological parameters and animal responses. A recent study (Ji et al. 2020b) has found the new indices (DHLI, ETIC) to predict heat stress no better than the older indices (THI, HLI, CCI). A more recent study (Lees et al. 2022) concluded the relative success of DHLI and THI in predicting heat stress depends on the physiological/behavioral indicator of interest (panting, drinking, standing) as well as the animals’ access to shade. The statistical indices discussed above consider the intensity of heat stress only. In other words, the duration of heat exposure (or relief) is not considered. Gaughan et al. (2008) have called this a “one-dimensional” approach. A few studies have attempted to incorporate the transient nature of the thermal interaction between animals and their surrounding, and specifically the effects of prolonged heat exposure and intermittent relief (e.g. at night). Relying on the classical THI index, Hahn and Mader (1997) proposed the hours above established THI thresholds (“THI-hours”) to be considered in the forecast of heat waves. Heinicke et al. (2018) have used a similar approach, based on THI and lying/standing behavior, to examine the effects of the duration of heat exposure in terms of a heat load duration (HLD) index. Similarly, Gaughan et al. (2008) used the length of the periods when the heat load index (HLI; see discussion in preceding paragraphs) is above or below a critical threshold to develop the Accumulated Heat Load (AHL) index. Methodologically, the AHL model offers notable advancement as it includes the effects of wind speed and solar radiation, which are absent from THI, as well as the effects of heat relief (HLI<threshold).
Although the premise of the “two-dimensional” heat load duration indices mentioned above is the consideration of heat balance (and heat accumulation in the case of bodily heat surplus), they are no different than the “one-dimensional" indices in their reliance on purely statistical correlations as a proxy for the thermal interaction between the animal and the environment. As pointed out by Ehrlemark and Sällvik (1996), it is a major shortcoming of the statistical approach that it ignores the thermodynamics of thermoregulation and heat dissipation. Ehrlemark and Sällvik (1996) found it therefore “not surprising” that practical experience from livestock management shows significant deviations from the predictions of the statistical models. Heat-balance models based on thermodynamic principles have the potential to address that shortcoming and complement statistical correlations between meteorological and physiological observations.
Heat-balance models for cattle
Research on heat-balance models for livestock has a history of more than three decades. Finding earlier thermal models, e.g. Porter and Gates (1969), limited in their incorporation of the thermoregulatory responses, McArthur (1987) developed a detailed steady-state heat-balance model for homeothermic vertebrates, which entailed the physiological responses. Despite the formal simplicity of the basic equation, the submodels used by McArthur (1987) to describe the underlying physical and physiological phenomena are rather complicated, entailing a high degree of non-linearity and coupling. Following the same general approach, Ehrlemark and Sällvik (1996) developed a steady-state heat-balance model. The ANIBAL (ANImal heat BALance) model (Ehrlemark and Sällvik 1996) is much simpler than the model by McArthur (1987) with potentially greater utility. Nevertheless, ANIBAL was not used to identify conditions of potential heat stress, but rather to predict heat generation at low (ambient) temperatures and evaporative heat loss at high (ambient) temperatures (Ehrlemark and Sällvik 1996). Moreover, while criticizing the traditional statistical approach for neglecting the effects of air speed, Ehrlemark and Sällvik (1996) used a similar index [the thermal load index (TLI); Ehrlemark and Sällvik (1996)] for normalizing the environmental conditions and comparison with experimental data and thus failed to address the shortcoming they had identified.
Acknowledging the practical difficulties arising from the complexity of McArthur’s model, Turnpenny et al. (2000a) presented a simplified version of the model, dubbed “parsimonious,” which was applied to various livestock [cattle, sheep, pigs, chickens (Turnpenny et al. 2000b)]. Following the same general approach, McGovern and Bruce (2000) developed a transient model which also included thermoregulatory mechanisms, namely reducing the thermal resistance of body tissue (vasodilation), sweating to increase latent heat loss and panting to increase respiratory heat loss. An accompanying algorithm for time-step simulations was also presented (McGovern and Bruce 2000). Similarly, Thompson et al. (2014) developed a comparable transient model, including relatively detailed climate submodels for calculating the ambient temperature, wind speed, and solar radiation, to be used when hourly weather data is not available. Li et al. (2021) added a submodel for conduction between a lying animal and the ground to the model by Thompson et al. (2014), further increasing its complexity. The model has so far only been used to perform a sensitivity analysis (Li et al. 2021). As mentioned above, none of these models has been systematically applied to identify conditions of potential heat stress, especially in terms of common meteorological parameters.
A general shortcoming of the mentioned heat-balance models is lack of validation against experimental data. Although most submodels used to describe individual thermal or physiological phenomena are well established, none of the whole models is thoroughly validated by comparison with reliable measurements. For instance, Ehrlemark and Sällvik (1996) compared the predictions of their model with experimental data pooled from several sources. Notably, the model predictions were in poor agreement with the experimental data at higher ambient temperatures, namely conditions of potential heat stress. Moreover, as mentioned above, the results were only presented in terms of a thermal load index (TLI) that obscures important boundary conditions such as the air speed. Recently, more attention has been paid to addressing the validation gap. Li et al. (2021), for example, compared their modeling results with experimental data from the literature, although with unsatisfactory results. The model overpredicts the core body temperature by up to 3°C which, given the physiological thresholds for heat stress, seems too large. On the other hand, the respiration rate, estimated based on linear regression with the body temperature, was underpredicted almost systematically, by up to 40% (60 br/min discrepancy at 140 br/min).
Turnpenny et al. (2000b) observed that the limited data available on the partition of heat loss, heat generation, and the thermophysical characteristics of the livestock hinders further development and refinement of heat-balance models. Two decades later, that limitation remains the case. Despite calorimetric methods, specifically measurements in respiration chambers, being well established and widely used for several decades, detailed measurements of heat transfer and thermoregulation in dairy cattle is scarce. In many widely used sources, the thermoneutral metabolic heat generation rate, a key boundary condition in heat-balance models, is calculated and reported as the residual value of energy-portioning calculations focusing on nutrition and productivity, e.g. Coppock (1985); van Knegsel et al. (2007); Talmón et al. (2020). On the other hand, thermodynamic measurements of thermoregulation which deal with the details of heat partition were mostly conducted decades ago, on animals with much lower yield than the present-day high-yielding cow, with presumably lower metabolic heat generation and possibly different thermophysiological characteristics. The seminal study by Worstell and Brody (1953), for example, was conducted on Holstein cows whose mean milk yield was less than 20 kg/day. The similarly widely referenced study by Purwanto et al. (1990) was conducted on cows among which the “high-yielding” group had a mean milk yield of about 30 kg/day. More recent measurements of respiratory and cutaneous heat losses by Maia et al. (2005a, b) were performed on pasture-fed Holsten cows with even lower yield, 15 kg/day on average. The existing models, including those referenced above, all rely on such historical data, while the modern Holstein-Friesian dairy cow can have yields averaging around 40 kg/day (Pinto et al. 2020) and exceeding 50 kg/day during peak lactation.
Perhaps driven by an increasing awareness of the endemic validation gap, there has recently been a slow resurgence of attention to the measurement of heat transfer and thermoregulatory responses in cattle. The recent thermographic study of the skin temperature by Yan et al. (2021) is a promising step forward, although the presentation of the results follows the THI orthodoxy. Another important development is the work of Zhou et al. (2021) where the physiological and productive responses of dairy cattle to various combinations of ambient temperature, humidity, and air speed were measured in a respiration chamber. Nevertheless, Zhou et al. (2021) do no present the partitioning of heat dissipation into different modes of heat transfer.
Another general disadvantage of the mechanistic heat-balance models is their formal complexity. Mechanistic models such as the heat-balance models discussed here have been criticized as unsuitable for use in precision farming due to complexity and presence of many parameters that often need to be re-evaluated or adjusted for each application (Wathes et al. 2008). Nevertheless, formal complexity and the resulting computational intensity are not necessarily as big a barrier to wide application as they were 20 or even 10 years ago. As pointed out by Stiehl and Marciniak-Czochra (2021), the present day’s computational power allows the investigation of rather complex issues based on mechanistic models, affording a deep quantitative understanding of a wide range of topics. Aside from the simplified general model by Turnpenny et al. (2000a, b), no effort has been made to address the implementation gap for heat-balance models of cattle, particularly to facilitate implementation in predictive-model control of the barn climate. Furthermore, as mentioned above, no attempt has been made at systematic application of such models to identify conditions of potential heat stress and develop meteorological indices.
Parallels with human biometeorology
Historical development
The ongoing research on heat stress in cattle exhibits several parallels with human biometeorology, specifically the development of human thermal comfort indices. Given the longer history and relatively greater success of the latter, there are lessons to be learned for a more effective pursuit of the former. After all, the most widely used index, THI, was imported from human meteorology, virtually shaping the trajectory of research on heat stress in cattle for more than half a century. The striking parallels between research on thermal comfort and heat stress in humans and cattle are therefore no coincidence. In both cases, researchers started with a focus on the ambient temperature, then developing statistical constructs that incorporated humidity, air speed, and finally solar radiation as wider ranges of climates and production facilities were considered. The parallels are demonstrated, for instance, by a recent study (Kovács et al. 2018) where the apparent temperature (for humans) was found to be a better predictor of heat stress in dairy calves than several variants of THI.
In 1962, about the time THI was imported into animal science, Macpherson (1962) published a comprehensive review of the metrics devised to assess thermal comfort in humans. Interestingly, Macpherson’s 1962 review enumerates 19 indices for human thermal comfort; Ji et al.’s 2020a review lists 20 heat stress indices for dairy cattle. As documented in by Macpherson (1962), the pioneering work on human thermal comfort was by large inspired and motivated by the proliferating industrial plant; industrial and mechanized animal husbandry has likewise enhanced the need for effective prediction and remediation of heat stress.
Judging from the large number of heat stress indices developed during the half century prior, Macpherson (1962) concluded that there was simultaneously a great need for quantifying and categorizing conditions of thermal comfort, and little success through the means thitherto devised. Most notably, Macpherson (1962) concluded:
“[A]ssessment of the thermal environment is not primarily a matter of the selection of some thermal index in which to express the results. Expressing the results in the form of an index may be a convenience, but the assessment of the environment is essentially the measurement of all the factors concerned… Indices of thermal stress do not provide a substitute for a sound knowledge of the mechanisms of heat exchange and of the physiological adjustments to the thermal environment.”
The current state of the art in dairy science suggests a similar conclusion.
Apparent temperature revisited
Inspired by the conclusion that a suitable heat index would be based on the heat balance of the human body (Macpherson 1962), Steadman (1979a, b, 1984) presented a meticulous analysis of heat transfer from the human body to derive an equivalent temperature. Dubbed the “apparent” temperature, this equivalent temperature is defined as the dry-bulb temperature at standardized humidity, wind speed, and radiation, which would require the same thermal resistance for a walking adult to feel thermal comfort under a given set of meteorological conditions. The procedure used to derive the apparent temperature, Tap, is as follows. The heat-balance equation is first solved iteratively for various sets of environmental conditions to obtain the thermal resistance of clothing, Rf, required for thermal balance. This first solution is based on the idea that, in equilibrium, postulated by Steadman (1979a) as a condition of thermal comfort, the total heat dissipation rate should equal the heat generation rate, less the net effect of the incoming solar, albedo and terrestrial radiation and the outgoing sky radiation (Steadman 1979a, b). Moreover, the core body temperature, Tb, is assumed constant at 37°C as a condition of comfort. After obtaining Rf, Tap is determined by solving the heat-balance equation for T, this time with Rf known from the first solution, and with the other meteorological parameters (solar radiation, air speed, and humidity) set as “standard,” i.e. reference, values.
Perhaps most relevant to the topic of heat stress in cattle and the slew of statistical indices is the demonstration (Steadman 1979b) that for any heat index, Tx, constructed as a linear combination of the dry-bulb temperature (Tdb), wet-bulb temperature (Twb), and globe temperature (Tgt), i.e.Footnote 2
the coefficients c1, c2, and c3 are not constant, but highly dependent on the ambient temperature and considerably dependent on activity level (heat generation), humidity, and wind speed (Steadman 1979b). This key observation explains the failure of indices constructed using regression analysis of environmental and physiological, behavioral, or productive parameters. The constant coefficients typically obtained from such analyses confine the proposed index to the environmental conditions and physiological characteristics covered in the original study. See for instance the recent work of Lees et al. (2022) which concluded that DHLI, which was developed to include the effects of solar radiation, is a better predictor of heat stress than THI (no radiation term), but only for unshaded cows. Ehrlemark and Sällvik (1996) duly observed that the validity of the statistical models is limited by the range of conditions covered by the underlying experimental data. Compare the general analytical expressions for c1, c2, and c3 derived by Steadman (1979b) and the definitions of common heat stress indices for cattle, summarized by Ji et al. (2020a).
Recognizing the need for simplified calculation of Tap, Steadman (1984) also presented linear equations based on multiple-regression analysis of computed values of Tap. There have subsequently been other efforts to develop simpler approximations that entail fewer independent variables, e.g. only Tdb and RH (Rothfusz 1990). It is important to note, however, that such regression-based equations are ultimately based on thermodynamic models, i.e. the heat balance of the animal, rather than on purely statistical correlations between observations of arbitrary predictors and indicators. Moreover, in constructing Tap, several conditions were imposed such that the result would have physical meaning and significance. For instance, it was observed that an equivalent temperature used to express comfort “must have the familiar properties of temperature” (Steadman 1984). As mentioned above, some of the cattle heat stress indices do not satisfy such criteria.
In general, Steadman’s model for humans is simpler than heat-balance models for cattle due to several underlying simplifying assumptions that are not applicable to cattle. In Steadman’s model, the basic link between the actual environmental conditions and the standard conditions at which Tap is evaluated is the thermal resistance of clothing (Rf). The fundamental assumption of the model is that, outdoors, humans seek (achieve) thermal comfort by adjusting clothing, more precisely the heat and moisture transfer resistance of clothing. This fundamental feature cannot be extended to animals, e.g. dairy cattle, since the haircoat is relatively constant, despite seasonal variations that decrease the heat and moisture transfer resistance of the coat in summer and vice versa in winter (Façanha et al. 2010). Furthermore, the assumptions and submodels used by Steadman (1979a, b) for evaluating the various heat and vapor transfer resistances do not apply to cattle. Lastly, it is important to note that the apparent temperature scale was developed based on a constant activity level, representing an adult Caucasian walking at 1.4 m/s (Steadman 1984). It must also be noted that research on human thermal comfort and perception goes on, with heat-balance models continuing to provide the framework for many influential developments. See, for instance, the review by Rupp et al. (2015).
Thermodynamic assessment of heat stress in dairy cattle
In this section, a model from the literature is applied following the general approach used to develop Tap to demonstrate the utility of heat-balance models in predicting heat stress, to derive meteorological indices, and to develop a general framework for systematic application of such models.
Assumptions and procedure
The general livestock heat-balance model developed by Turnpenny et al. (2000a, b) was used where the total heat dissipation from the animal, Ge, is estimated and compared with the thermoneutral metabolic heat generation rate, M, both expressed in terms of heat flux, per unit skin surface area. In general, Ge is comprised of sensible and latent heat loss from the skin and through respiration. With no solar radiation, thermal balance (equilibrium) is maintained when Ge=M.
The thermoregulatory responses are iteratively adjusted to find conditions where Ge=M, following the “principle of least metabolic cost” (Mount 1974; Turnpenny et al. 2000a), namely that an animal will use vasomotor control before increasing evaporative heat loss which involves water loss and/or an increase in metabolic rate. This means that, for given boundary conditions, thermoregulation is simulated by:
-
1)
Decreasing the tissue resistance to heat transfer (rs), simulating vasodilation, until thermal balance is achieved (Ge=M). There is a physiological lower limit to this resistance.
-
2)
If the minimum tissue resistance is not sufficient for thermal balance, i.e. Ge<M, the cutaneous latent heat loss (sweating) is increased until thermal balance is achieved. There is an upper limit to this heat loss mechanism, dictated by either physiology or the environment.
-
3)
In the present model, the respiration rate was independently calculated, based on the ambient temperature and humidity and using empirical correlations.
-
4)
If the maximum sweating rate is not sufficient to maintain balance, i.e. Ge<M, heat will accumulate in the body and the core body temperature will increase.
Note that, as pointed out by McArthur (1987), there is evidence that sweating starts before tissue resistance has been minimized, i.e. thermoregulation through sweating and vasodilation may occur simultaneously and not necessarily in succession. Nevertheless, the step-by-step model of Turnpenny et al. (2000a) provides a reasonable first approximation.
Further note that:
-
Metabolic heat generation was assumed constant at the thermoneutral rate. In reality, the metabolic rate declines with prolonged exposure to heat, thereby increasing the animal’s tolerance to heat. This adjustment is achieved by reduced food intake and thyroid gland activity. See the paper by McArthur (1987) and the references therein cited for details.
-
Similarly, the core body temperature, Tb, was assumed constant at the thermoneutral level (39°C).
-
As will be shown, the skin temperature is a dependent variable, i.e. not directly adjusted as a thermoregulatory response.
-
Only the onset of bodily heat accumulation is sought. Therefore, the increase in Tb and reduction of M as secondary coping mechanisms were not modeled.
Some of the sub-models used here are slightly different from those used by Turnpenny et al. (2000a, b). Details of the model implementation including boundary conditions, submodels, and validation against experimental data are presented in the Appendix.
Sample modeling results
Figure 1 shows sample results obtained from running the heat-balance model at sample constant air speed and relative humidity, and for various values of the ambient temperature, Ta. The main output to observe is Ge, specifically its magnitude relative to M. As mentioned above, bodily heat accumulation starts when Ge<M. Latent heat flux from the skin is denoted by Ec while the respiratory heat flux (dominantly latent) is denoted by Er. In the present model, Er is a function of the Ta and RH only, i.e. adjusted independently of vasodilation and sweating. See the Appendix for details. The third component of Ge, not shown in Fig. 1, is the sensible heat flux from skin (convection and long-wave radiation).
Equilibrium heat fluxes and skin temperature of a Holstein dairy cow as a function of the ambient temperature and for various air speed and relative humidities and no solar radiation, estimated based on the heat-balance model of Turnpenny et al. (2000a). M, metabolic heat generation; Ge, total heat dissipiation; Ec, cutaneous latent heat loss; Er, repsiratory heat loss; Ts, skin temperature. Shaded area denotes Ge<M where heat accumulates in the body and Tb=const and M=const assumptions may no longer be valid
Figure 1a suggests that, for u=2 m/s and RH=40%, the thermoregulatory responses are sufficient to maintain the heat balance up to just above 20°C. In other words, increasing Ta to ~20°C, vasodilation and sweating are able to reduce the overall heat transfer resistance between the body core and the ambient in order to compensate for the reduction in Tb-Ta. The thermoregulatory responses in this region (Ta ≲ 20.5°C) can be divided to two phases:
-
1)
For Ta ≲ 8°C, vasodilation is sufficient for maintaining Ge=M, as seen from the increase in the skin temperature, Ts, plotted against the vertical axis on the right. (Because M and Tb are constant, reducing rs increases Ts; see Eq. (A4) in the Appendix.)
-
2)
After rs reaches its physiological minimum, i.e. vasodilation exhausted, sweating is enhanced to dissipate more latent heat from the skin; this is reflected by the monotonic increase of Ec for 8°C ≲ Ta ≲ 20.5°C. The linear increase in Ec counteracts the linear decrease of the sensible heat flux, caused by the decrease in the heat transfer potential (Tb-Ta). The slope of both lines is the convection heat transfer coefficient, proportional to a power of the air speed. See Eq. (A6) in the Appendix.
For u=2 m/s and RH=40%, the onset of heat accumulation at Ta≈20.5°C is dictated by Ec reaching its limit, in this case Ec,max=120 W/m2. As discussed in the Appendix, this limit can be physiological or environmental.
In reality, once rs=rs,min and Ec=Ec,max, Tb increases, followed by a decrease in M due to reduced feed intake and metabolic activityFootnote 3. The results shown in Fig. 1a are therefore only qualitatively valid for Ta ≳ 20.5°C, nonetheless instructive. The shaded area denotes Ge<M.
The effect of air speed can be seen from comparison of Fig. 1a, b, and d. Higher air speed, corresponding to a higher convective heat loss from the skin, shifts the onset of heat accumulation to a higher Ta. In other words, vasodilation (reflected by the rise in Ts) and sweating (reflected by the rise in Ec) are triggered and therefore exhausted at higher Ta, meaning Ge=M can be maintained for higher values of Ta.
Most notably, Fig. 1 suggests that humidity has little effect on the onset of heat accumulation. Compare Fig. 1b and c, representing moderate (RH=40%) and extremely high (RH=90%) humidity, respectively. Note how the various heat fluxes are virtually identical up to Ta≈27°C, well beyond the onset of heat accumulation (Ta≈15°C in both cases). Even at u=0.5 m/s, corresponding to “clam” air (WMO 1970), Ec reaching its physiological limit is the determining factor. The adverse effect of excessive humidity (RH=90%; Fig. 1c) on heat dissipation becomes apparent only at Ta ≈27°C, some 12°C above the onset of heat accumulation, where Ec is suppressed below the physiological limit, leading to a drastic drop in Ge. This is in agreement with the conclusion by Turnpenny et al. (2000b) that evaporative heat loss from cattle is restricted by ambient vapor pressure only at high ambient temperatures (Ta>30°C), while at lower ambient temperatures, sweating is limited by water supply rather than environmental conditions. Similarly, the effect of humidity on Er is only significant for Ta ≳ 30°C, as seen from Fig. 1a and c.
From heat fluxes to temperatures
As shown above, the main outcome of heat-balance models is heat dissipation fluxes which can then be compared to fluxes from the heat sources (metabolic heat generation and solar irradiation) to assess thermal balance. Nevertheless, heat fluxes are not as tangible as meteorological parameters (ambient temperature, humidity, air speed). Moreover, as discussed in Heat stress: Indicators and predictors, effective implementation for heat stress prevention/alleviation depends on indices and thresholds in terms of the readily available meteorological parameters. This section presents three proposals for translating the heat-flux results into simplified indices for the state of thermoregulation and the onset of heat accumulation.
Onset of heat accumulation: the critical temperature
In order to express the heat-balance results in terms of the more familiar meteorological parameters, a critical temperature, Tcr, may be defined as the ambient temperature corresponding to the onset of heat accumulationFootnote 4 for given u and RH. Here, Tcr was defined as Ta for which Ge falls to 99% of M, an arbitrary threshold.
In Fig. 2, Tcr is plotted as a function of u for RH=40% and three representative values of the physiological limit on Ec, denoted by \({\hat{E}}_{\mathrm{c},\max }\):
-
1)
\({\hat{E}}_{\mathrm{c},\max }=120\ \mathrm{W}/{\mathrm{m}}^2\) corresponding to historical data for Holstein cows, used by Turnpenny et al. (2000b), also the default value in the present model.
-
2)
\({\hat{E}}_{\mathrm{c},\max }=138\ \mathrm{W}/{\mathrm{m}}^2\) corresponding to the average of the measurements by Gebremedhin et al. (2010) for Holstein cows.
-
3)
\({\hat{E}}_{\mathrm{c},\max }=200\ \mathrm{W}/{\mathrm{m}}^2\) corresponding to the upper end of the measurements by Gebremedhin et al. (2010). This is an extremely high heat flux, unlikely to be sustained over the entire skin area, especially at M = 240 W/m2. This value was nonetheless included for demonstration and comparison purposes.
Recall that, as discussed in the “Sample modeling results” section, humidity hardly has any effect on the onset of heat accumulation. The significant effect of air speed (u), on the other hand, can be seen in Fig. 2, especially for \({\hat{E}}_{\mathrm{c},\max }=120\ \mathrm{W}/{\mathrm{m}}^2\) and \({\hat{E}}_{\mathrm{c},\max }=138\ \mathrm{W}/{\mathrm{m}}^2\): increasing the air speed from 0.5 to 6 m/s, shifts Tcr by as much as 10°C. For \({\hat{E}}_{\mathrm{c},\max }=200\ \mathrm{W}/{\mathrm{m}}^2\), the excessive sweating capacity can compensate reduced convective heat loss at lower air speeds to a large extent, diminishing the difference between Tcr at u=0.5 m/s and u=6 m/s. Noteworthy is that, according to Fig. 2, heat accumulation may start well below the established values for the upper critical temperature (UCT), e.g. 25°C according to Berman et al. (1985), especially for u < 4 m/s. The Tcr results are in agreement with UCT=25°C only for animals with extremely high sweating capacity (\({\hat{E}}_{\mathrm{c},\max }=200\ \mathrm{W}/{\mathrm{m}}^2\)) or at high air speeds (u > 4 m/s).
Apparent temperature for cattle
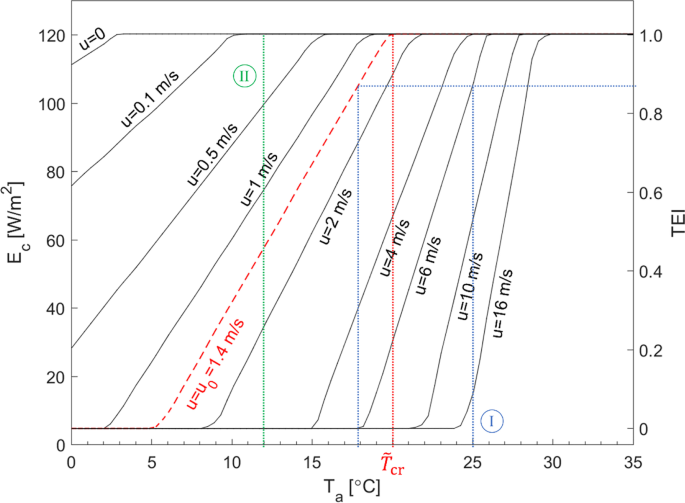
The next step in consolidating the results would be to integrate T and u into a single parameter. Following the general procedure used by Steadman (1979a, b, 1984) to develop Tap, an “apparent temperature” for cattle, \({\overset{\sim}{T}}_{\mathrm{ap}}\), may be defined for any combination of Ta and u as the ambient temperature which would require the same Ec for thermal equilibrium at a reference air speed.Footnote 5 The main difference with Tap (Steadman 1984) is that, instead of the required clothing insulation, Ec is the link between the actual and apparent temperatures. Since air is rarely still in naturally ventilated dairy barns, u0=1.4 m/s was chosen as the reference air speed, corresponding to the upper end of the “light air” range in the Beaufort scale (WMO 1970) and in accordance with the original Tap (Steadman 1984; see the “Apparent temperature revisited” section).
The procedure for calculating \({\overset{\sim }{T}}_{\mathrm{ap}}\) is demonstrated in Fig. 3 where the equilibrium Ec is plotted as a function of Ta and for various values of u. For any given combination of Ta and u, there is a unique equilibrium Ec so long as Ta<Tcr. To find \({\overset{\sim }{T}}_{\mathrm{ap}}\), the corresponding line of constant Ec is intersected with the u0 curve (dashed curve in Fig. 3); \({\overset{\sim }{T}}_{\mathrm{ap}}\) is the abscissa of the intersection.
Equilibrium cutaneous latent heat flux (Ec) and thermoregulatory exhaustion index (TE) as functions of ambient temperature and at various air speeds; graphical evaluation of apparent temperature for cattle (\({\overset{\sim }{T}}_{\mathrm{ap}}\)) [M = 240 W/m2, \({\hat{E}}_{\mathrm{c},\max }\) = 120 W/m2, no solar radiation]
Even in the absence of strong winds, there is air movement around caused by free convection and the movements of the animals. The u=0 curve was therefore included in Fig. 3 only as a reference for comparison. On the other hand, u=16 m/s represents “near gale” high winds (WMO 1970), also included for comparison. In most applications, the air speed is between 0.5 and 6 m/s.
Thermoregulatory exhaustion index
From Fig. 2, it is known that Tcr =20°C for u=1.4 m/s and Ec,max = 120 W/m2. Transformed into \({\overset{\sim }{T}}_{\mathrm{ap}}\), any combination of Ta and u can simply be compared against this threshold, \({\overset{\sim }{T}}_{\mathrm{cr}}={T}_{\mathrm{cr}}\left({u}_0\right)={20}^{{}^{\circ}}\mathrm{C}\), to determine whether thermonetural heat balance can be maintained as well as how far the conditions from the onset of heat accumulation are. A second integrated parameter can be defined to characterize the extent to which the thermoregulatory mechanisms (particularly sweating) have been “exhausted” and as a measure of how far the conditions from the upper limit of the thermoneutral zone are. Here, the thermoregulatory exhaustion index (TEI) is defined as:
where Ec,min and Ec,max denote the minimum and maximum of Ec, respectively.Footnote 6
One advantage of TEI over Tcr is that it entails two universal limits, TEI=0 and TEI=1.0 correspoding to Ec= Ec,min and Ec= Ec,max respectively. In other words, TEI consolildates the effect of the most important enviornmental (Ta, u) and phsyiological parameters (Ec,max). TEI=1.0 signifies the exhaustion of the “primiary” thermoregulatory respnse mechanisms and the onset of heat accumulation.
In Fig. 3, TEI is plotted against the vertical axis on the right. Two examples are also shown:
In Example I, \({\overset{\sim }{T}}_{\mathrm{ap},\mathrm{I}}<{\overset{\sim }{T}}_{\mathrm{cr}}\), meaning Ge=M can be sustained and therefore thermoneutrality maintained, reflected by TEII<1.0. On the other hand, in Example II, Ec = Ec,max, meaning the thermoregulatory reponses are exausted (TEIII = 1.0) beyond thermoneutrality and heat accumulation is underway.
Note that the results shown in Fig. 3 apply to the representative values of the physiological parameters, particularly M and \({\hat{E}}_{\mathrm{c},\max }\), used in the present model. Similar graphs can be generated for animals with different characteristics or other weather conditions of interest, including with solar radiation.
Conclusion
Indices constructed through regression of meteorological parameters and animal responses have dominated research on heat stress in cattle for more than six decades. Nevertheless, there is increasing skepticism about the effectiveness and adequacy of such indices. As suggested by the parallels with human biometeorology drawn in this paper, forging new paths forward to meet the needs of modern livestock management in the times of climate change requires a renewed attention to physics-based heat-balance models. Although several heat-balance models have been developed, no attempt has been made at systematic application of the models to predict conditions of potential heat stress.
In that context, the present work revisited classical work in human biometeorology to develop a framework for identifying heat stress based on thermodynamic models of thermoregulation and heat dissipation. A model from the literature was used to assess the heat balance of a typical Holstein dairy cow under various combinations of ambient temperature, humidity, and air speed. It was shown that the onset of heat accumulation strongly depends on temperature and air speed, but hardly on humidity. While many studies have paid little attention to the effect of air speed on heat stress, especially in the vicinity of the animal, the results of the present study underline the importance of systematic collection and reporting of air speed data.
As evidenced by the dominance and resurgence of THI and its many variants, an easy-to-use index presented in tables or simple graphs is extremely useful. Simplicity and ease-of-use compel practitioners and researchers to be surprisingly forgiving of fundamental and methodological shortcomings. Therefore, the present work introduced new parameters to translate the modeling results (heat fluxes) into meteorological indices. The critical temperature denotes the onset of heat accumulation at given air speed. The apparent temperature for cattle maps the ambient temperature at any given air speed onto a thermophysiologically equivalent temperature at a reference air speed. Finally, the thermoregulatory exhaustion index (TEI) is a measure of the extent to which the thermoregulatory responses have been mobilized and how far the conditions from the upper limit of the thermoneutral zone are.
The general framework developed in this paper can serve as a roadmap for future work. To further establish the thermodynamic approach, the endemic validation gap must be first addressed through detailed measurements of the thermophysiological characteristics of the modern high-yielding cow, most importantly the thermoneutral metabolic heat generation rate and core boy temperature, and the maximum sweating rate. Furthermore, concrete definitions for what constitutes heat stress must be developed in physiological or productive terms. More precisely, thresholds for critical heat strain, e.g. in terms of increase in the core body temperature or decrease in milk yield, must be established. Finally, metabolism and productivity must be integrated in the thermodynamic heat dissipation models in order to increase the utility and accuracy of the models for analysis at animal-individual level.
Notes
The empirical wind speed data used to derive the correction were in the range 0.6–15.5 m/s (Mader et al. 2010).
Note that indices defined in terms of T and RH, e.g. THI, can be recast in terms of Tdb and Twb (c3=0) using psychrometric relations or curve-fits.
M may initially increase due to panting but will eventually drop in response to prolonged heat exposure (McArthur 1987).
Blaxter and Wainman (1962) have similarly used the term “critical temperature” in assessing cold stress in steers to denote the temperature below which metabolism is increased to maintain the normal body temperature.
Mader et al. (2010) have similarly used “apparent temperature” as an alternative term for the Comprehensive Climate Index (CCI), defined as the dry-bulb temperature with three linear corrections for the effects of humidity, wind speed and radiation.
See the Appendix for more details about each limit.
Abbreviations
- E c :
-
Cutaneous latent heat flux [W/m2]
- \({\hat{E}}_{\mathrm{c},\max }\) :
-
Physiological limit on cutaneous latent heat flux [W/m2]
- E r :
-
Respiratory heat flux [W/m2]
- G e :
-
Total heat dissipation flux [W/m2]
- M :
-
Metabolic heat generation flux [W/m2]
- RH:
-
Relative humidity [%]
- T a :
-
Ambient temperature [°C]
- T b :
-
Body temperature [°C]
- T ap :
-
Apparent temperature [°C]
- \({\overset{\sim }{T}}_{\mathrm{ap}}\) :
-
Apparent temperature for cattle [°C]
- T cr :
-
Critical temperature [°C]
- T s :
-
Skin temperature [°C]
- TEI:
-
Thermoregulatory exhaustion index [-]
- THI:
-
Temperature humidity index [-]
- u :
-
Air speed [m/s]
References
Berman A (2003) Effects of body surface area estimates on predicted energy requirements and heat stress. J Dairy Sci 86(11):3605–3610. https://doi.org/10.3168/jds.S0022-0302(03)73966-6
Berman A (2004) Tissue and external insulation estimates and their effects on prediction of energy requirements and of heat stress. J Dairy Sci 87(5):1400–1412. https://doi.org/10.3168/jds.S0022-0302(04)73289-0
Berman A (2005) Estimates of heat stress relief needs for Holstein dairy cows. J Anim Sci 83(6):1377–1384. https://doi.org/10.2527/2005.8361377x
Berman A (2006) Extending the potential of evaporative cooling for heat-stress relief. J Dairy Sci 89:3817–3825. https://doi.org/10.3168/jds.S0022-0302(06)72423-7
Berman A, Folman YM, Kaim M, Mamen Z, Herz D, Wolfenson A, Graber Y (1985) Upper critical temperatures and forced ventilation effects for high-yielding dairy cows in a tropical climate. J Dairy Sci 68:488–495
Bianca W (1962) Relative importance of dry-and wet-bulb temperatures in causing heat stress in cattle. Nature 195:251
Blaxter KL, Wainman FW (1962) Environmental temperature and the energy metabolism and heat emission of steers. J Agric Sci 56:81–90
Blaxter KL, Wainman FW (1964) The effect of increased air movement on the heat production and emission of steers. J Agric Sci 62:207–214
Bloomberg M, Bywater AC (2007) Estimating the effect of shade on heat stress in New Zealand dairy cows using two published models. MODSIM07 - Land, Water and Environmental Management: Integrated Systems for Sustainability, Proceedings.
Cena K, Monteith JL (1975) Transfer processes in animal coats. 3. Water vapour diffusion. Proc R Soc Lond B 188:413–423
Coppock CE (1985) Energy nutrition and metabolism of the lactating dairy cow. J Dairy Sci 68:3403–3410
dos Santos MM, Souza-Junior JBF, Dantas MRT et al (2021) An updated review on cattle thermoregulation: physiological responses, biophysical mechanisms, and heat stress alleviation pathways. Environ Sci Pollut Res 28:30471–30485. https://doi.org/10.1007/s11356-021-14077-0
Ehrlemark AG, Sällvik KG (1996) A model of heat and moisture dissipation from cattle based on thermal properties. Transactions of the ASAE 39(1):187–194
Façanha DAE, da Silva RG, Maia ASC, Guilhermino MM, de Vasconcelos AM (2010) Annual variation of morphologic traits and haircoat surface temperature of Holstein cows in semi-arid environment. R Bras Zootec 39(4):837–844
Gaughan JB, Mader TL, Holt SM, Lisle A (2008) A new heat load index for feedlot cattle. J Anim Sci 86(1):226–234. https://doi.org/10.2527/jas.2007-0305
Gaughan JB, Mader TL, Holt SM, Sullivan ML, Hahn GL (2010) Assessing the heat tolerance of 17 beef cattle genotypes. Int J Biometeorol 54;6:617–627. https://doi.org/10.1007/s00484-009-0233-4
Gebremedhin KG, Lee CN, Hillman PE, Collier RJ (2010) Physiological responses of dairy cows during extended solar exposure. Trans ASABE 53(1):239–247
Godyń D, Herbut P, Angrecka S (2019) Measurements of peripheral and deep body temperature in cattle – a review. J Therm Biol 79:42–49. https://doi.org/10.1016/j.jtherbio.2018.11.011
Hahn GL, Mader TL (1997) Heat waves in relation to thermoregulation, feeding behaviour and mortality of feedlot cattle. Proc. 5th Int. Livest. Environ. Symp., Bloomington [Ed: MN. R. W. Bottcher and S. J. Hoff], pp. 563–571. Am Soc Agric Eng, St. Joseph, MI.
Heinicke J, Hoffmann G, Ammon C, Amon B, Amon T (2018) Effects of the daily heat load duration exceeding determined heat load thresholds on activity traits of lactating dairy cows. J Therm Biol 77:67–74. https://doi.org/10.1016/j.jtherbio.2018.08.012
Ji B, Banhazi T, Perano K, Ghahramani A, Bowtell L, Wang C, Li B (2020a) A review of measuring, assessing and mitigating heat stress in dairy cattle. Biosyst Eng 199:4–26. https://doi.org/10.1016/j.biosystemseng.2020.07.009
Ji B, Banhazi T, Perano K, Ghahramani A, Bowtell L, Wang C, Li B (2020b) Modelling of heat stress in a robotic dairy farm. Part 1: thermal comfort indices as the indicators of production loss. Biosyst Eng 199:27–42. https://doi.org/10.1016/j.biosystemseng.2019.11.004
Kamal R, Dutt T, Patel M, Dey A, Chandran PC, Bharti PK, Barari SK (2016) Behavioural, biochemical and hormonal responses of heatstressed crossbred calves to different shade materials. J Appl Anim Res 44:347–354. https://doi.org/10.1080/09712119.2015.1074076
van Knegsel AT, van den Brand H, Dijkstra J, van Straalen WM, Heetkamp MJ, Tamminga S, Kemp B (2007) Dietary energy source in dairy cows in early lactation: energy partitioning and milk composition. J Dairy Sci 90(3):1467–1476. https://doi.org/10.3168/jds.S0022-0302(07)71632-6
Kovács L, Kézér FL, Ruff F, Szenci O, Jurkovich V (2018) Association between human and animal thermal comfort indices and physiological heat stress indicators in dairy calves. Environ Res 166:108–111. https://doi.org/10.1016/j.envres.2018.05.036
Le Cozler Y, Allain C, Xavier C, Depuille L, Caillot A, Delouard JM, Delattre L, Luginbuhl T, Faverdin P (2019) Volume and surface area of Holstein dairy cows calculated from complete 3D shapes acquired using a high precision scanning system: interest for body weight estimation. Comput Electron Agric 165:104977. https://doi.org/10.1016/j.compag.2019.104977
Lee DHK (1965) Climatic stress indices for domestic animals. Int J Biometeorol 9:29
Lees J, Lees A, Gaughan J (2018) Developing a heat load index for lactating dairy cows. Anim Prod Sci 58(8):1387–1391
Lees JC, Lees AM, Gaughan JB (2022) The influence of shade availability on the effectiveness of the Dairy Heat Load Index (DHLI) to predict lactating cow behavior, physiology, and production traits. Int J Biometeorol 66:289–299. https://doi.org/10.1007/s00484-021-02186-x
Li J, Narayanan V, Kebreab E, Dikmen S, Fadel JG (2021) A mechanistic thermal balance model of dairy cattle. Biosyst Eng 209:256–270. https://doi.org/10.1016/j.biosystemseng.2021.06.009
van der Linden A, Ven GWJ, Oosting S, Ittersum MK, Boer I (2018) LiGAPS-Beef, a mechanistic model to explore potential and feed-limited beef production 1: model description and illustration. Animal 13:1–11. https://doi.org/10.1017/S1751731118001726
Macpherson RK (1962) The assessment of the thermal environment. A review. Br J Ind Med 19(3):151–164. https://doi.org/10.1136/oem.19.3.151
Mader TL, Johnson LJ, Gaughan JB (2010) A comprehensive index for assessing environmental stress in animals. J Anim Sci 88(6):2153–2165
Madhusoodan AP, Seijian V, Rashamol VP, Savitha ST, Bagath M, Krishnan G, Bhatta R (2019) Resilient capacity of cattle to environmental challenges – an updated review. J Animal Behav Biometeorol 7:104–118. https://doi.org/10.31893/2318-1265jabb
Maia ASC, da Silva RG, Loureiro CB (2005a) Sensible and latent heat loss from the body surface of Holstein cows in a tropical environment. Int J Biometeorol 50(1):17–22. https://doi.org/10.1007/s00484-005-0267-1
Maia ASC, da Silva RG, Loureiro CMB (2005b) Respiratory heat loss of Holstein cows in a tropical environment. Int J Biometeorol 49(5):332–336. https://doi.org/10.1007/s00484-004-0244-0
McArthur AJ (1987) Thermal interaction between animal and microclimate: a comprehensive model. J Theor Biol 126:203–238
McGovern RE, Bruce JM (2000) Model of the thermal balance for cattle in hot conditions. J Agric Eng Res 77(1):81–92. https://doi.org/10.1006/jaer.2000.0560
Monteith JL, Unsworth MH (1990) Principles of Environmental Physics, 2nd edn. Arnold, London
Mount LE (1974) Thermal neutrality. In: Monteith JL, Mount LE (eds) Heat loss from animals and man. Butterworths, London, pp 205–231
Pinto S, Hoffmann G, Ammon C, Amon T (2020) Critical THI thresholds based on the physiological parameters of lactating dairy cows. J Therm Biol 88:102523. https://doi.org/10.1016/j.jtherbio.2020.102523
Porter WP, Gates DM (1969) Thermodynamic equilibria of animals with environment. Ecol Monogr 39:227–244
Purwanto BP, Abo Y, Sakamoto R, Furumoto F, Yamamoto S (1990) Diurnal patterns of heat production and heart rate under thermoneutral conditions in Holstein Friesian cows differing in milk production. J Agric Sci 114(2):139e142. https://doi.org/10.1017/S0021859600072117
Ratnakaran A.P., Sejian V., Jose V.S., Vaswani S., Bagath M., Krishnan G., Beena V.., Indira DP., Varma G., Bhatta R. (2017). Behavioural responses to livestock adaptation to heat stress challenges. Asian Australas J Anim Sci 11:1–13. https://doi.org/10.3923/ajas.2017.1.13.
Rothfusz L.P. (1990). The heat index ‘equation’ (or, more than you ever wanted to know about heat index). Technical Attachment. Scientific Services Division, NWS Southern Region Headquarters, Fort Worth, TX. SR 90-23, 7/1/90.
Rupp RF, Vásquez NG, Lamberts R (2015) A review of human thermal comfort in the built environment. Energy Build 105:178–205. https://doi.org/10.1016/j.enbuild.2015.07.047
Sejian V, Bhatta R, Gaughan JB, Dunshea FR, Lacetera N (2018) Review: adaptation of animals to heat stress. Animal 12:431–444. https://doi.org/10.1017/S1751731118001945
da Silva RG, Maia ASC (2011) Evaporative cooling and cutaneous surface temperature of Holstein cows in tropical conditions. R Bras Zootec 40(5):1143–1147
Spiers DE (2012) Physiological basics of temperature regulation in domestic animals. In: Environmental Physiology of Livestock, First Edition. Edited by R.J. Cillier & J.L. Collier. John Wiley Sons. P.17-34.
Steadman RG (1979a) The Assessment of Sultriness. Part I: A Temperature-Humidity Index Based on Human Physiology and Clothing Science. J Appl Meteorol Climatol 18(7):861–873. https://doi.org/10.1175/1520-0450(1979)018%3C0861:TAOSPI%3E2.0.CO;2
Steadman RG (1979b) The Assessment of Sultriness. Part II: Effects of Wind, Extra Radiation and Barometric Pressure on Apparent Temperature. J Appl Meteorol Climatol 18(7):874–885. https://doi.org/10.1175/1520-0450(1979)018%3C0874:TAOSPI%3E2.0.CO;2
Steadman RG (1984) A universal scale of apparent temperature. J Appl Meteorol Climatol 23(12):1674–1687. https://doi.org/10.1175/1520-0450(1984)023%3C1674:AUSOAT%3E2.0.CO;2
Stevens DG (1981) A model of respiratory vapor loss in Holstein dairy cattle. Trans ASAE 24(1):0151–0153. https://doi.org/10.13031/2013.34215
Stiehl T., Marciniak-Czochra A. (2021) Intelligente Algorithmen und Gleichungen? – Eine Annäherung an die Intelligenz mathematischer Konzepte. Ausgabe: Intelligenz – Theoretische Grundlagen und praktische Anwendungen (Holm-Hadulla R.M., Funke J., Wink M.). https://doi.org/10.17885/heiup.hdjbo.2021.1.24389. [In German]
Talmón D, Garcia-Roche M, Mendoza A, Mattiauda DA, Carriquiry M (2020) Energy partitioning and energy efficiency of two Holstein genotypes under a mixed pasture-based system during mid and late lactation. Livest Sci 239:104166. https://doi.org/10.1016/j.livsci.2020.104166
Thom EC, Bosen JF (1959) The discomfort index. Weatherwise 12:57–60
Thompson VA, Barioni LG, Rumsey TR, Fadel JG, Sainz RD (2014) The development of a dynamic, mechanistic, thermal balance model for Bos indicus and Bos taurus. J Agric Sci 152:464–482. https://doi.org/10.1017/S002185961300049X
Turnpenny JR, McArthur AJ, Clark JA, Wathes CM (2000a) Thermal balance of livestock – 1. A parsimonious model. Agric For Meteorol 101:15–27
Turnpenny JR, Wathes CM, Clark JA, McArthur AJ (2000b) Thermal balance of livestock – 2. Applications of a parsimonious model. Agric For Meteorol 101:29–52
Wang X, Gao H, Gebremedhin KG, Bjerg BS, van Os J, Tucker CB, Zhang G (2018) A predictive model of equivalent temperature index for dairy cattle (ETIC). J Therm Biol 76:165–170
Wathes CM, Kristensen HH, Aerts J-M, Berckmans D (2008) Is precision livestock farming an engineer’s daydream or nightmare, an animal’s friend or foe, and a farmer’s panacea or pitfall? Comput Electron Agric 64(1):2–10. https://doi.org/10.1016/j.compag.2008.05.005
Webster AJF (1974) Heat loss from cattle with particular emphasis on the effects of cold. In: Monteith JL, Mount LE (eds) Heat Loss from Animals and Man. Butterworths, London, pp 205–231
West JW (2003) Effects of Heat-Stress on Production in Dairy Cattle. J Dairy Sci 86:2131–2144
Wiersma F, Nelson GL (1967) Nonevaporative heat transfer from the surface of a bovine. Transactions of the ASAE 10(6):733–737
World Meteorological Organization (WMO) (1970) Commission for maritime meteorology. The Beaufort Scale of Wind Force. (Technical and Operational Aspects). Geneva, WMO.
Worstell DM, Brody S (1953) Environmental physiology and shelter engineering. XX. Comparative physiological reactions of European and Indian cattle to changing temperature. Missouri agric, exp. Sta Res Bull 515
Yan G, Liu K, Hao Z, Li H, Shi Z (2021) Development and evaluation of thermal models for predicting skin temperature of dairy cattle. Comput Electron Agric 188:106363. https://doi.org/10.1016/j.compag.2021.106363
Zhou M, Aarnink AJA, Huynh TTT, van Dixhoorn IDE, Groot Koerkamp PWG (2021) Effects of increasing air temperature on physiological and productive responses of dairy cows at different relative humidity and air velocity levels. J Dairy Sci Article in press. https://doi.org/10.3168/jds.2021-21164
Funding
This work was supported by funding received from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 101000785. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Appendix: Details of the heat balance model
Appendix: Details of the heat balance model
General approach and formulation
The heat-balance model by Turnpenny et al. (2000a,b) was implemented where the total heat dissipation from the animal, Ge, is estimated, and compared with the thermoneutral metabolic heat generation, M. When the skin surface is in thermal equilibrium, the total heat flux dissipated to the environment equals the heat flux through the body tissue, Gs, as well as the heat flux through the haircoat, Gc;
For given environmental conditions (ambient temperature, humidity, air speed, solar radiation), heat-balance equations are solved iteratively to find the equilibrium skin and coat temperatures. The thermoregulatory responses are incrementally increased during iterations until converged. See the papers by Turnpenny et al. (2000a, b) for more details and general solution algorithm.
Cattle dissipate bodily heat mainly through respiration and from skin. The total heat dissipation rate on a flux (per unit area) basis can be written as shown in Eq. (A1) (Turnpenny et al. 2000a).
where Ge is the heat flux per unit skin surface area; the first term on the right-hand side represents convective heat transfer from the haircoat to the ambient air; the second term represents the net long-wave radiant exchange at the external surface of the haircoat; Sabs is the absorbed solar radiation; Er is the dominantly latent heat flux through respiration; and Ec is the latent heat flux from the skin, both normalized by the skin surface are, As.
Following McArthur (1987), the formulation of Monteith and Unsworth (1990) was used for the sensible components of heat loss:
where ΔT is the driving temperature difference, r [s/m] is the resistance to heat transfer, and ρcp is constant, here taken as 1220 J/(m3K), corresponding to 20°C and 1 atm.
Animal data
The animal size characteristics were chosen to represent a typical mature Holstein cow: m=670 kg, dt=0.5 m. A haircoat length of l =9 mm was chosen to represent the summer conditions (Façanha et al. 2010).
The skin surface area, As [m2], was calculated using the regression proposed by Webster (1974):
The result is in agreement with the measurements of Le Cozler et al. (2019) though not with the conclusion by Berman (2003).
The model by Turnpenny et al. (2000b) was originally developed as a generic model for livestock, including sheep, pigs, cattle, and poultry. Consequently, the model entails parameters to account for the difference between the skin surface area and the haircoat surface area as well as the haircoat’s effect on the curvature of the exposed surface. Given that l is small for cattle, those two effects were ignored in the present work. Furthermore, the entire skin area was assumed to be exposed, representing a cow in standing position and thermally isolated from other animals. The latter assumption is supported by the conclusion by Wiersma and Nelson (1967) that convective heat loss is not affected by the presence of animals farther than two inches (approximately five centimeters).
The metabolic heat flux, M [W/m2], was calculated based on the regression proposed by van Knegsel et al. (2007) for daily heat production in lactating cows, 1110 m0.75 [kJ], resulting in an average metabolic heat flux of M=240 W/m2. More recent measurements by Talmón et al. (2020) yield comparable results for pasture-fed Holstein cows.
Following Turnpenny et al. (2000b), the core body temperature was assumed constant at Tb=39°C.
Ambient conditions
Ambient conditions were defined in terms of pressure, p [kPa], temperature, Ta [°C], mean radiant temperature, Tr [°C], relative humidity, RH [%], wind speed, u [m/s], and assuming no solar irradiation. For simplicity it was assumed Tr=Ta, though it is likely that Tr >Ta during hot sunny days.
Heat transfer through body tissue
The rate of heat transfer through body tissue is given by:
where rs [s/m] is the resistance of the body tissue to heat transfer, Tb is the core body temperature, and Ts is the mean temperature of the skin surface.
The vasomotor response is simulated by adjusting rs, starting at 100 s/m at the start of each iteration and decreasing to a minimum of 30 s/m, before the sweating rate is adjusted. The maximum and minimum values of rs were chosen based on the work of Webster (1974) and Turnpenny et al. (2000b).
Heat loss from skin
Convective heat loss
Convective heat transfer between the outer surface of the haircoat and the ambient air can be written as:
Here, the convective resistance was estimated using the correlation by Wiersma and Nelson (1967), reproduced in Eq. (A6), with both the Reynolds number (Re) and Nusselt number (Nu) based on the trunk diameter, set in the present model to dt=0.5 m.
Radiant heat exchange
Since heat stress in cattle sheltered in naturally ventilated barns is of main interest to the present study, the radiant exchange calculations were simplified by assuming no solar radiation (Sabs=0) and considering only the long-wave radiant exchange with the surrounding surfaces. The net long-wave radiant exchange between the outer surface of the haircoat and the surroundings, L, can be written as shown in Eq. (A7) with the radiation resistance rR obtained from the linearized Stefan’s Law.
Latent heat loss from skin
Heat loss through the evaporation of sweat on the skin (Ec [W/m2]) is a crucial heat dissipation mechanism, and its maximum a major determinant of the onset of heat stress. There is a physiological limit on the sweating rate, dictated by water availability and the activity of sweat glands (McArthur 1987). As pointed out by McArthur (1987) and Turnpenny et al. (2000b), Ec,max is normally determined by this physiological limit. Turnpenny et al. (2000b) used \({\hat{E}}_{\mathrm{c},\max }=120\ \mathrm{W}/{\mathrm{m}}^2\) as the physiological limit, derived from studies conducted in the 1970s. It is likely that the modern high-yield cow has a higher sweating capacity. A 2010 study (Gebremedhin et al. 2010), for instance, reports Ec,max as high as 280 W/m2 from cattle subject to hot and dry conditions in the shade, though the reported mean is less than half the maximum (138 W/m2). Maia et al. (2005a) have similarly reported measurements of Ec well exceeding 300 W/m2 for pasture-fed Holstein cows in a tropical climate, which is difficult to reconcile with typical metabolic heat generation rates (M<300 W/m2), especially for the relatively low-yielding cows (~15 kg/day) studied in that work. A 2011 study of Holstein cows (da Silva and Maia 2011), on the other hand, reports Ec < 100 W/m2. In the present work, unless otherwise specified, \({\hat{E}}_{\mathrm{c},\max }=120\ \mathrm{W}/{\mathrm{m}}^2\) was assumed, in accordance with Turnpenny et al. (2000b).
In high humidity, Ec may be supressed below the physiological limit due to low evaporation potential from the skin. As suggested in by Turnpenny et al. (2000b), this environmental limit can be estimated as:
where e [Pa] is the vapor pressure, γ is the psychrometric constant (0.066 kPa/K), and rv [s/m] is the lower limit of the resistance to mass transfer, namely the vapor transfer resistance due to diffusion and free convection through the haircoat, given by Cena and Monteith (1975) as:
where D [m2/s] is the diffusivity of water vapor in air (2.5×10−5 m2/s at 20°C), and Ts* and Ta* [K] are the virtual temperature of skin and air, respectively. The virtual temperature is defined in Eq. (A10) (McArthur 1987).
To account for the effect of wind on the evaporation of sweat, l in Eq. (A9) is replaced with l–lw where lw is the “wind penetration depth” (Turnpenny et al. 2000a).
In general, Ec,max must be re-evaluated in each iteration as the smaller of the physiological and environmental limits;
Under conditions of primary interest to the present study (Ta ≤ 40°C, RH<60%), \({\overset{\sim }{E}}_{\mathrm{c},\max }>120\)W/m2 and the physiological limit of 120 W/m2 prevails.
As suggested by McArthur (1987), the evaporation of moisture from an animal’s body can take place below the skin surface. In cold, for instance, when the sweat glands are inactive and the skin surface is dry, there is water vapor loss by diffusion through the skin. Therefore, Ec,min>0. According to McArthur (1987), Ec,min ≈ 0.04Ec,max in homeotherms. This baseline was implemented in the present model by initializing Ec at 0.04Ec,max.
Respiratory heat loss
Respiratory heat loss was calculated based on an energy balance between the inspired and expired air streams. The inspired air was assumed to be at the ambient conditions, i.e. re-inhalation of the expired air was ignored. The expired air was assumed to be saturated at a temperature calculated based on the empirical correlation proposed by Stevens (1981):
As noted by Stevens (1981), the correlation above has two important implications:
-
1)
The exhaled air is not at the body temperature, as assumed in many studies, e.g. McArthur (1987), Turnpenny et al. (2000a, b) and McGovern and Bruce (2000).
-
2)
Tex is a function of the ambient conditions only.
The respiration rate, R [br/min], and tidal volume, Vt [m3/br], were also calculated using correlations proposed by Stevens (1981):
Note the reverse trends of Vt and R as Ta increases, i.e. rapider but shallower respiration at higher Ta. As noted by Stevens (1981), the inspired air cools the upper respiratory tract as it passes through. When air is expired, on the other hand, it is cooled by the respiratory passages. At a given Ta, increasing RH reduces the cooling potential of the inhaled air. Therefore, the upper respiratory tract is cooled by the inhaled air to a lesser extent, leading to higher Tex.
The correlations above apply to first-phase panting only (Stevens 1981; Berman 2004). Furthermore, the correlations were obtained based on environmental chamber measurements with no induced air flow. Equation (A13) is therefore expected to overestimate R in open barns where there is virtually always air flow. An overestimated R will however only lead to a more conservative estimation of heat stress. Moreover, Er is by far dominated by Ec, making the overestimation of Er even less problematic.
Alternatively, the comparable correlations proposed by Maia et al. (2005b) can be used to evaluate the respiratory heat loss as a function the ambient temperature only.
Validation
In the absence of detailed heat-transfer measurements on the modern, high-yielding dairy cattle, data from the seminal work of Blaxter and Wainman (1964) on steers was used for validating the present implementation of the model by Turnpenny et al. (2000a,b). The measurements by Blaxter and Wainman (1964) were divided into seven groups of three trials based on the air temperature and speed. To simulate each group, the mean values were used to define the boundary conditions (Ta, u) and animal characteristics (m, l, M, Tb, rs,min) in the model. See Table 1. The ambient humidity and surface temperatures were not reported by Blaxter and Wainman (1964). In the model, a moderate humidity of RH=40% was assumed. Based on data from an earlier study using the same respiration chamber (Blaxter and Wainman 1962), it was assumed that Tr=Ta.
In Fig. A1, the model predictions for the skin temperature (Ts) and total evaporative (latent) heat loss (E) are compared to the measurements (Blaxter and Wainman 1964). The horizontal error bars represent the two-standard-deviation band of the three trials in each group. The model predictions are in overall agreement with the measurements, particularly in the case of Ts. In the absence of data that can be used for a more thorough validation, the comparison shown in Fig. A1 was taken as sufficient validation of the model.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Foroushani, S., Amon, T. Thermodynamic assessment of heat stress in dairy cattle: lessons from human biometeorology. Int J Biometeorol 66, 1811–1827 (2022). https://doi.org/10.1007/s00484-022-02321-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-022-02321-2